The transvaginal mesh is a synthetic or biological material utilized in reconstructive pelvic surgeries to provide support for weakened or damaged tissues, available in various types and serving distinct purposes.

Definition and Purpose
Transvaginal mesh stands as a medical device, often used to treat pelvic organ prolapse and stress urinary incontinence in women.
Its primary function involves providing additional support to weakened or damaged tissues around the bladder, rectum, and uterus.
The use of this surgical mesh intends to address significant health concerns like discomfort or embarrassment caused by these conditions.
However, it’s also crucial to note that transvaginal permanent mesh is linked with lower instances of repeat surgery compared to native tissue repair, even though its usage corresponds with higher rates of potential complications.
Types of Transvaginal Mesh
Three primary types of transvaginal mesh exist.
Polypropylene mesh, the most frequently used type, is a synthetic option featuring large pores and monofilament construction which allows tissue growth through its spaces.
Absorbable or biological mesh, made from animal tissues such as cowhide or pigskin, is another option – it gradually dissolves and encourages new tissue growth in patients.
Lastly, composite meshes are available; these hybrids boast polypropylene layers combined with absorbable coating that prevents exposure to surrounding organs.
Each type suit different needs based on the patient’s condition and surgeon’s preference.
When is Transvaginal Mesh Used?
Transvaginal mesh is primarily utilized in surgeries for treating conditions such as pelvic organ prolapse and stress urinary incontinence among women.
Pelvic Organ Prolapse Surgery
Pelvic organ prolapse surgery is an operation to address a condition where one or more pelvic organs descend into the vagina.
This medical issue often affects women after childbirth, as pregnancy and delivery can weaken the muscles that support these organs.
Surgeons have historically used transvaginal mesh in this procedure to provide extra support.
The mesh, typically made of synthetic plastic, serves as a sturdy scaffold for your body’s tissues to grow around.
However, recent studies indicate higher rates of complications with this surgical approach versus others not involving transvaginal mesh.
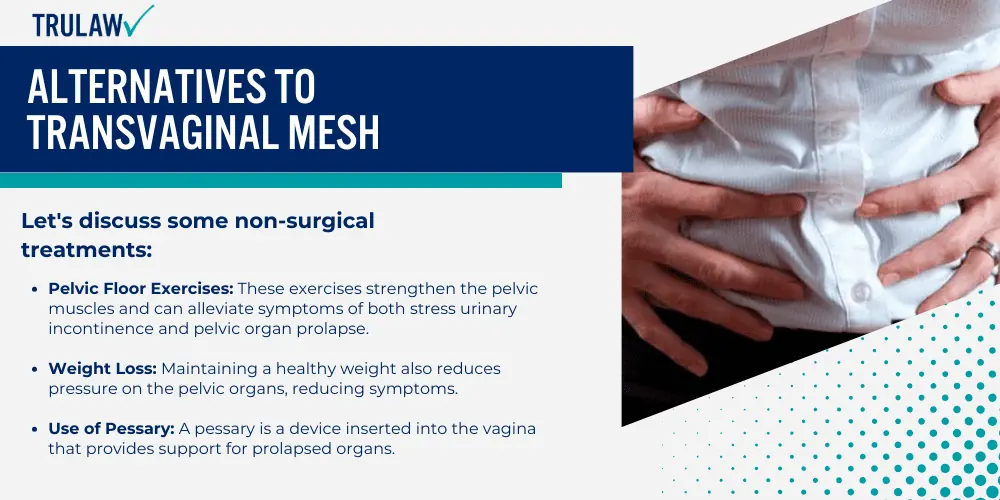
As such concerns grow, surgeons are now exploring alternative treatments, both surgical and non-surgical for pelvic organ prolapse.
Stress Urinary Incontinence Surgery
Stress urinary incontinence surgery is a procedure primarily used when non-surgical treatment options fail to improve this condition.
Surgeons often utilize transvaginal mesh for support during the operation, including the commonly performed midurethral sling procedure.
The goal of these surgical interventions is to provide an effective and long-term solution for women experiencing stress urinary incontinence—a common issue characterized by involuntary leakage of urine during physical activities such as coughing or sneezing.
However, it’s essential to know that permanent mesh implementation may come with higher rates of de novo stress incontinence and serious complications reported by the FDA, such as erosion and bladder sling issues.