Statute of Limitations for Transvaginal Mesh Lawsuits
- Last Updated: July 14th, 2025

Attorney Jessica Paluch-Hoerman, founder of TruLaw, has over 28 years of experience as a personal injury and mass tort attorney, and previously worked as an international tax attorney at Deloitte. Jessie collaborates with attorneys nationwide — enabling her to share reliable, up-to-date legal information with our readers.
Legally Reviewed
This article has been written and reviewed for legal accuracy and clarity by the team of writers and legal experts at TruLaw and is as accurate as possible. This content should not be taken as legal advice from an attorney. If you would like to learn more about our owner and experienced injury lawyer, Jessie Paluch, you can do so here.
Fact-Checked
TruLaw does everything possible to make sure the information in this article is up to date and accurate. If you need specific legal advice about your case, contact us by using the chat on the bottom of this page. This article should not be taken as advice from an attorney.
Key takeaways:
- The FDA issued multiple safety warnings about transvaginal mesh complications from 2008 to 2019, leading to a surge in lawsuits against major manufacturers like Johnson & Johnson and Boston Scientific.
- Transvaginal mesh lawsuits have been consolidated into multidistrict litigations (MDLs), with bellwether trials resulting in significant plaintiff victories and influencing subsequent global settlements.
- Potential claimants must be aware of state-specific statutes of limitations and eligibility criteria based on the onset of complications.
Overview of Transvaginal Mesh Lawsuits
On this page, we’ll provide an overview of Transvaginal Mesh Lawsuits, the legal basis for transvaginal mesh claims, recent developments of ongoing transvaginal mesh litigation, and much more.

Intro to Transvaginal Mesh Lawsuits
Some of the key aspects of transvaginal mesh lawsuits include, but are not limited to:
- Product Liability Claims: Many mesh lawsuits allege that the devices were defectively designed, manufactured, or marketed.
- Failure to Warn: Plaintiffs often claim that manufacturers failed to adequately warn patients and doctors about the risks of mesh implants.
- Statute of Limitations: The deadline for filing a mesh lawsuit varies by state, making it important to act promptly to preserve your legal rights.
- Multidistrict Litigation (MDL): Many transvaginal mesh lawsuits have been consolidated into MDLs to streamline legal processes and promote efficient resolution.
If you’ve suffered complications from a transvaginal mesh implant, you may be entitled to significant compensation through a mesh lawsuit.
Contact TruLaw using the chat on this page for a free case evaluation to learn more about your legal rights and the process of filing a transvaginal mesh claim.
Table of Contents
FDA Safety Warnings and the Rise of Transvaginal Mesh Lawsuits
Faced with mounting adverse event reports, the FDA issued several safety communications regarding the risks associated with transvaginal mesh products, leading to a surge in related lawsuits.

FDA’s Public Health Notifications on Transvaginal Mesh Risks
The Food and Drug Administration (FDA) has actively monitored the safety of transvaginal mesh devices that repair pelvic organ prolapse (POP).
Here’s a timeline of the FDA’s actions regarding transvaginal mesh:
- In 2008, the FDA released a public health notification highlighting the complications related to transvaginal mesh after receiving over 1,000 adverse reports within three years.
- 2011 saw the FDA upgrading the previous alert, clarifying that mesh-related complications are not rare, which was a significant shift in stance from 2008.
- According to the press announcements on their website, the FDA brought Specialists and patient groups together in 2019 to discuss the safety and efficacy of these devices.
- The agency mandated the reclassification of transvaginal mesh for POP repair as Class III, which denotes high risk and required manufacturers to submit premarket approval applications.
Explosion of Transvaginal Mesh Lawsuits Following FDA Warnings
The increased transparency and severity of FDA warnings did not go unnoticed by the public or the legal community.
They triggered a sharp increase in transvaginal mesh lawsuits, reflecting patients’ growing concerns:
- In response to the FDA warnings, individuals who experienced severe complications from vaginal mesh implants began seeking legal retribution.
- Law firms nationwide started representing thousands of women complaining of mesh-related complications in mesh lawsuits.
- The influx of lawsuits increased awareness and scrutiny of medical devices, especially pelvic mesh products.
These FDA actions and subsequent legal battles highlight an ongoing concern regarding the safety of medical devices and the vigilance required to ensure patient well-being.
Major Transvaginal Mesh Manufacturers Facing Lawsuits
In recent years, several medical device companies have faced legal challenges due to the serious complications associated with transvaginal mesh products.

These lawsuits have highlighted the significant health risks posed to patients and have resulted in substantial settlements.
List of Medical Device Companies Producing Transvaginal Mesh
These companies are among those that manufactured and marketed transvaginal mesh and have since faced legal action over concerns about their products.

The following manufacturers have produced transvaginal mesh and are now confronting litigation:
- Johnson & Johnson and its subsidiary Ethicon recently agreed to a major settlement for their marketing practices.
- Boston Scientific Corporation, another key player, settled for a significant amount to resolve allegations of deceptive marketing.
- California Department of Justice announced that Boston Scientific Corporation agreed to a multistate settlement for the deceptive marketing of its surgical mesh products.
- The Washington Attorney General’s Office also secured a settlement in which Boston Scientific will pay over eight million for its transvaginal mesh products.
Allegations in Transvaginal Mesh Lawsuits Against Manufacturers
Thousands of women have filed suit against manufacturers of transvaginal mesh, alleging the products caused serious health problems.
Legal cases against mesh manufacturers often involve several allegations, including:
- The manufacturers did not properly warn about the risks associated with their surgical mesh products.
- Claims that the mesh devices were defectively designed, contributing to a high incidence of serious complications.
- Assertions that companies engaged in deceptive marketing tactics, overstating the effectiveness and safety of the vaginal mesh products.
- Numerous patients have experienced significant health issues post-implantation, leading to a wave of vaginal mesh cases.
Transvaginal Mesh MDLs and Bellwether Trials
Multidistrict litigation (MDL) and a series of bellwether trials have significantly shaped the management and outcomes of transvaginal mesh litigation.

The MDL process has consolidated many cases to streamline pretrial proceedings, while bellwether trials have guided the resolution of thousands of similar claims.
Consolidation of Federal Transvaginal Mesh Lawsuits into MDLs
Multidistrict litigation has played a pivotal role in addressing the numerous lawsuits filed against manufacturers of transvaginal mesh devices.
Key facts about this process include:
- The Judicial Panel on Multidistrict Litigation consolidated federal lawsuits involving transvaginal mesh into several MDLs to improve efficiency.
- These MDLs have been established in various federal district courts, with a significant concentration in the Southern District of West Virginia.
- The transvaginal mesh MDL has been one of the largest in history, encompassing thousands of individual cases alleging injuries from the pelvic mesh implants.
- Through consolidation, common issues of fact and law can be addressed collectively while allowing individual cases to retain their unique aspects.
Significant Plaintiff Victories in Bellwether Trials 2012-2019
Bellwether trials serve as early test cases and profoundly affect litigation by providing insights into how juries might respond to evidence and testimony.
Notable achievements in these trials include:
- Major mesh verdicts have underscored the credibility of plaintiffs’ claims, with several resulting in multimillion-dollar awards.
- From 2012 to 2019, these bellwether trials often concluded with significant settlements and verdicts favoring the plaintiffs.
- Bellwether outcomes have influenced subsequent transvaginal mesh settlements, sometimes prompting manufacturers to offer to resolve ongoing litigation.
- These verdicts and settlements provided compensation for women who experienced severe complications due to the pelvic mesh implant, including pain, infection, and organ damage.
Global Settlements and Resolution of Transvaginal Mesh MDLs
In the wake of widespread litigation concerning transvaginal mesh products, manufacturers have faced numerous lawsuits leading to substantial settlements and the resolution of multidistrict litigations (MDLs).

Manufacturers Entering Global Settlements Following Bellwethers
The transvaginal mesh litigation saw significant activity when bellwether trials provided critical outcomes that influenced large-scale settlements.
These bellwether cases serve as test trials that help parties gauge how juries might respond to evidence and testimony in similar lawsuits.
As a result of these bellwether trials, several manufacturers entered into global settlements:
- Boston Scientific agreed to pay an eight-figure sum to resolve allegations surrounding their mesh products.
- Johnson & Johnson and their subsidiary, Ethicon, were involved in a major settlement of over $116.9 million addressing claims of deceptive marketing practices.
- C.R. Bard, another prominent manufacturer, settled claims as seen in the California Department of Justice press release.
- Companies have resolved mesh settlement claims totaling hundreds of millions, reflecting some of the largest transvaginal mesh settlements.
Majority of 100,000+ Cases Resolved, MDLs Deactivated
The sheer number of transvaginal mesh cases filed led to the creation of multiple MDLs to manage these claims efficiently.
Many of these cases have since been resolved, deactivating many MDL dockets.
Here’s a breakdown of how these cases were addressed:
- Over 100,000 transvaginal mesh cases were consolidated under MDLs across the United States.
- The resolution of these cases involved substantial mesh settlements, compensating affected women.
- MDLs were largely successful in managing and resolving the influx of earlier vaginal mesh lawsuits.
- MDLs were deactivated as cases were settled or went to trial, marking a milestone in the lengthy litigation process.
Current Status of Transvaginal Mesh Litigation in 2023
In 2023, the landscape of transvaginal mesh litigation remains active, with continued legal action and critical deadlines impacting new cases.
Continued Filing of New Transvaginal Mesh Lawsuits
Individuals continue to bring forth new lawsuits against manufacturers of transvaginal mesh products.
These legal actions assert that the devices caused complications such as infection, pain, and organ perforation.
Significant litigation developments include:
- Ongoing settlement negotiations with major manufacturers.
- Heightened awareness among potential claimants has led to an influx of cases.
- California and Washington have an active role in multistate settlements, like the one against Boston Scientific Corporation for deceptive marketing practices.
- An increase in individual claims outside of mass tort proceedings.
Challenges Posed by Statutes of Limitations for New Claims
The deadline to file a lawsuit, known as the statute of limitations, is a decisive factor for new claims.
This varies by state, impacting the eligibility of potential lawsuits.
Essential points regarding statutes of limitations:
- Statutes of limitations can differ greatly from one state to another, affecting the timing of filing a new lawsuit.
- Claimants must be vigilant in filing claims within these deadlines to preserve their legal rights.
- In some states, the discovery rule may extend the time limit to file, depending on when the injury was, or should have been, discovered.
- Legal counsel is often sought to navigate these time constraints to avoid forfeiting the right to sue.
It remains essential for individuals affected by vaginal mesh maker complications to seek timely legal advice considering these constraints and the ongoing nature of the litigation.
Eligibility Criteria for Filing a Transvaginal Mesh Lawsuit
When considering a vaginal mesh lawsuit for complications related to transvaginal mesh, understanding the eligibility criteria is essential.
Two primary considerations include the onset of complications and adherence to state-specific time constraints for filing.
Recent Onset of Transvaginal Mesh Complications as Key Factor
Complications may arise at different times for patients with transvaginal mesh implants.
Onset timing is a key point of consideration in determining eligibility for litigation.
Symptoms such as mesh erosion and organ perforation can be particularly impactful.
Individuals should consider these crucial signs as potential prompts for legal action:
- Mesh erosion or exposure leading to pain or discomfort
- Infections that are hard to treat and persist over time
- Organ perforation and the accompanying sharp pains
- Recurring pelvic organ prolapse or incontinence issues
These often indicate a product’s failure and can establish a foundation for claiming personal injury.
State-Specific Statutes of Limitations for Personal Injury Claims
Each state’s laws govern the time limits for filing personal injury claims.
In pelvic mesh cases, the statutes of limitations are pivotal in determining if a new vaginal mesh lawsuit can proceed.
The following list illustrates time-sensitive considerations for potential claimants:
- The time frame usually begins once the patient becomes aware of the complications.
- The window of opportunity can range from one year to several years, depending on the state.
- In some instances, exceptions can extend these deadlines, specific to medical complications.
- Legal advice is strongly recommended to navigate these time restrictions effectively.
Familiarity with these statutes and their application to an individual’s circumstances is imperative when pursuing legal recourse for transvaginal mesh injuries.
Estimated Settlement Values for Transvaginal Mesh Lawsuits
When examining the settlement values of transvaginal mesh lawsuits, it’s evident that outcomes depend on individual vaginal mesh case details and the extent of patients’ complications.

Common Complications Associated with Transvaginal Mesh Implants
Transvaginal mesh implants have been associated with a range of complications, leading to significant settlements.
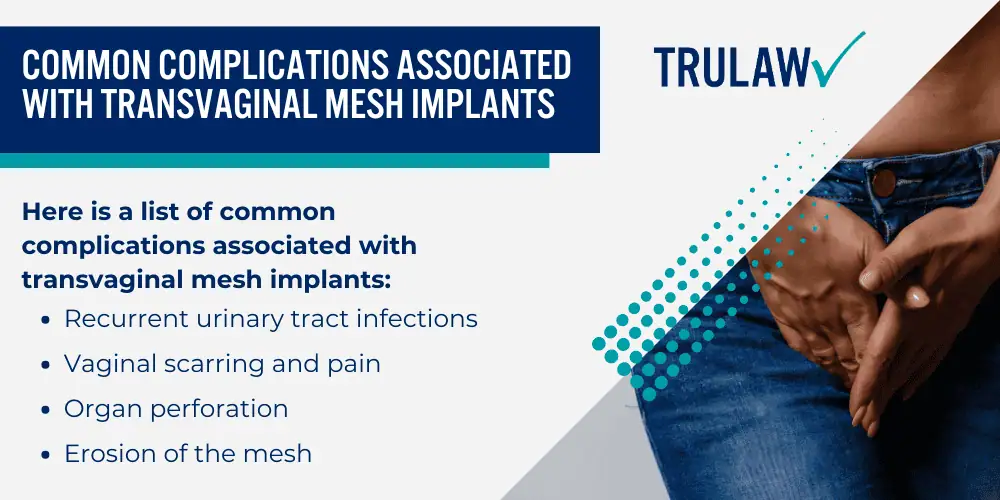
Here is a list of common complications associated with transvaginal mesh implants:
- Recurrent urinary tract infections
- Vaginal scarring and pain
- Organ perforation
- Erosion of the mesh
These medical complications often necessitate additional surgeries, increasing patient suffering and medical expenses.
Factors Influencing Transvaginal Mesh Settlement Payouts
Many factors influence a woman’s compensation in a transvaginal mesh lawsuit.
Settlement payouts for transvaginal mesh lawsuits can vary widely, influenced by several key factors:
- The severity of the complications experienced
- The extent of additional medical treatments required, including surgeries
- Whether there’s a need for lifelong medical care as a consequence of the implant
- Lost wages and the impact on the patient’s ability to earn in the future
- The level of documented pain and suffering
Transvaginal mesh settlements also account for the manufacturer’s conduct, such as failing to warn about potential risks.
In cases where manufacturers have been found to have engaged in particularly egregious behavior, punitive damages may also be awarded.
Transvaginal Mesh Lawsuit Frequently Asked Questions
-
Recent legal proceedings have seen substantial settlements for claimants.
Notably, Washington residents received details on obtaining their share of a $9.9 million recovery after the state sued Johnson & Johnson for failing to disclose the risks of their surgical mesh devices.
In another case, Johnson & Johnson and Ethicon, Inc. agreed to a $116.9 million settlement over deceptive marketing allegations, with the investigation spanning 41 states and the District of Columbia.
-
Compensation for bladder mesh lawsuits can vary significantly based on numerous factors, but it typically ranges from thousands to millions of dollars.
The exact amount typically relates to the severity of injuries, the number of surgeries required, and the impact on the claimant’s quality of life.
-
Every state has its statute of limitations ranging from 1 to 6 years, generally starting from the date the claimant either underwent mesh surgery or discovered the complications related to the mesh.
It is imperative to consult a vaginal mesh lawyer promptly, as these time frames are a crucial determinant of claim eligibility.
-
Eligibility for a claim is typically established based on criteria like having had the mesh implanted, suffering complications or injuries, and meeting the lawsuit’s filing deadlines.
Consulting with an experienced attorney is important for precise eligibility assessment.
-
When seeking an attorney, potential claimants should look for experience with vaginal mesh device litigation, a history of successful settlement outcomes, readiness to go to trial if necessary, and a transparent fee structure.
It’s also beneficial to select someone who communicates clearly and compassionately.
-
Frequent complications encouraging lawsuits are chronic pain, infection, mesh erosion, and organ perforation.
Lawsuits also cite instances of urinary problems and the need for additional surgeries to remove or adjust the mesh.

Managing Attorney & Owner
With over 25 years of legal experience, Jessica Paluch-Hoerman is an Illinois lawyer, a CPA, and a mother of three. She spent the first decade of her career working as an international tax attorney at Deloitte.
In 2009, Jessie co-founded her own law firm with her husband – which has scaled to over 30 employees since its conception.
In 2016, Jessie founded TruLaw, which allows her to collaborate with attorneys and legal experts across the United States on a daily basis. This hypervaluable network of experts is what enables her to share the most reliable, accurate, and up-to-date legal information with our readers!
Additional Transvaginal Mesh Lawsuit resources on our website:
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?
At TruLaw, we fiercely combat corporations that endanger individuals’ well-being. If you’ve suffered injuries and believe these well-funded entities should be held accountable, we’re here for you.
With TruLaw, you gain access to successful and seasoned lawyers who maximize your chances of success. Our lawyers invest in you—they do not receive a dime until your lawsuit reaches a successful resolution!
AFFF Lawsuit claims are being filed against manufacturers of aqueous film-forming foam (AFFF), commonly used in firefighting.
Claims allege that companies such as 3M, DuPont, and Tyco Fire Products failed to adequately warn users about the potential dangers of AFFF exposure — including increased risks of various cancers and diseases.
Depo Provera Lawsuit claims are being filed by individuals who allege they developed meningioma (a type of brain tumor) after receiving Depo-Provera birth control injections.
A 2024 study found that women using Depo-Provera for at least 1 year are five times more likely to develop meningioma brain tumors compared to those not using the drug.
Suboxone Tooth Decay Lawsuit claims are being filed against Indivior, the manufacturer of Suboxone, a medication used to treat opioid addiction.
Claims allege that Indivior failed to adequately warn users about the potential dangers of severe tooth decay and dental injuries associated with Suboxone’s sublingual film version.
Social Media Harm Lawsuits are being filed against social media companies for allegedly causing mental health issues in children and teens.
Claims allege that companies like Meta, Google, ByteDance, and Snap designed addictive platforms that led to anxiety, depression, and other mental health issues without adequately warning users or parents.
Transvaginal Mesh Lawsuits are being filed against manufacturers of transvaginal mesh products used to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
Claims allege that companies like Ethicon, C.R. Bard, and Boston Scientific failed to adequately warn about potential dangers — including erosion, pain, and infection.
Bair Hugger Warming Blanket Lawsuits involve claims against 3M — alleging their surgical warming blankets caused severe infections and complications (particularly in hip and knee replacement surgeries).
Plaintiffs claim 3M failed to warn about potential risks — despite knowing about increased risk of deep joint infections since 2011.
Baby Formula NEC Lawsuit claims are being filed against manufacturers of cow’s milk-based baby formula products.
Claims allege that companies like Abbott Laboratories (Similac) and Mead Johnson & Company (Enfamil) failed to warn about the increased risk of necrotizing enterocolitis (NEC) in premature infants.
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?
