Ozempic Lawsuit | Ozempic and Wegovy Claims
- Last Updated: July 3rd, 2025

Attorney Jessica Paluch-Hoerman, founder of TruLaw, has over 28 years of experience as a personal injury and mass tort attorney, and previously worked as an international tax attorney at Deloitte. Jessie collaborates with attorneys nationwide — enabling her to share reliable, up-to-date legal information with our readers.
Legally Reviewed
This article has been written and reviewed for legal accuracy and clarity by the team of writers and legal experts at TruLaw and is as accurate as possible. This content should not be taken as legal advice from an attorney. If you would like to learn more about our owner and experienced injury lawyer, Jessie Paluch, you can do so here.
Fact-Checked
TruLaw does everything possible to make sure the information in this article is up to date and accurate. If you need specific legal advice about your case, contact us by using the chat on the bottom of this page. This article should not be taken as advice from an attorney.
Key takeaways:
- Ozempic and Wegovy, used for diabetes management and weight loss, are under investigation for causing stomach paralysis.
- An Ozempic Lawsuit and other legal actions are being explored for patients who suffered stomach paralysis after using these drugs.
- While no FDA warnings exist for Ozempic and Wegovy, there are warnings for their compounded versions.
Ozempic Lawsuit Overview
On this page we’ll discuss the potential of an Ozempic Lawsuit, what drugs like Ozempic and Wegovy are designed to do, side effects associated with Ozempic, who may qualify for an Ozempic Lawsuit, and much more.
Intro to the Ozempic Lawsuit & Stomach Paralysis Claims
Ozempic is an FDA approved drug originally used to improve blood sugar in adults with type 2 diabetes, but has been popularize for weight loss.
Wegovy is an FDA approved drug used for chronic weight management in adults with obesity.
These drugs, or compounded versions of these drugs, may be linked to severe gastroparesis (stomach paralysis), gallbladder disease, cyclic vomiting syndrome, and other serious medical conditions.

Stomach paralysis is a condition affecting nerves and muscles in the stomach, interfering with the process that moves food through the stomach and into the small intestine.
Symptoms and side effects of stomach paralysis can be painful, uncomfortable, and may lead to long-term health problems.
Gallbladder disease involves conditions like gallstones and inflammation (cholecystitis), which can cause discomfort and may require gallbladder removal.
Legal action is being investigated for these symptoms and injuries.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
Table of Contents

Ozempic Lawsuit Updates Timeline
A growing wave of lawsuits is being filed against Novo Nordisk, alleging that its GLP-1 receptor agonist medications—including Ozempic and Wegovy—caused permanent vision loss due to non-arteritic anterior ischemic optic neuropathy (NAION).
These new claims are currently being pursued in courts across the country as standalone cases and are not part of the existing multidistrict litigation (MDL) in the Eastern District of Pennsylvania, which is focused solely on gastrointestinal injuries such as gastroparesis and intestinal blockages.
The uptick in NAION-related lawsuits follows the publication of a June 2025 study in JAMA Ophthalmology, which found a significant association between semaglutide use and increased risk of optic nerve damage.
Plaintiffs argue that Novo Nordisk failed to warn about the risk of sudden and irreversible vision impairment, while the company continues to deny any causal connection and has not updated its product labels to include vision loss as a potential side effect.
With the number of vision-related cases growing and adverse event reports mounting, legal observers are questioning whether the current GLP-1 MDL could be expanded—or if a separate MDL might be created—to address these additional injuries.
For now, however, the vision loss lawsuits remain outside the existing MDL framework.
The Ozempic multidistrict litigation (MDL) has expanded to 1,997 cases as of July 2025, marking an increase from 1,882 cases reported in June.
Plaintiffs allege that Ozempic and similar GLP-1 receptor agonist drugs have caused serious gastrointestinal complications, including gastroparesis (stomach paralysis), intestinal blockages, and chronic vomiting.
The court is currently overseeing the early stages of the litigation, including discussions around bellwether trial selection and the structure of discovery.
Plaintiffs are pushing for access to internal safety data and records of adverse events, while the defense is expected to challenge the scientific link between the medications and alleged injuries.
If you or a loved one experienced severe digestive issues—such as stomach paralysis, bowel obstruction, or ileus—after using Ozempic, you may be eligible to pursue legal action.
Contact the experienced Ozempic Lawyers at TruLaw for a free consultation, or use the chatbot on this page to check your eligibility for an Ozempic Lawsuit instantly.
U.S. District Judge Mark Pittman has upheld the FDA’s decision to remove semaglutide—the active ingredient in Ozempic and Wegovy—from its drug shortage list, effectively ending compounding pharmacies’ ability to produce versions of the popular weight-loss medications.
The lawsuit, brought by the Outsourcing Facilities Association, challenged the FDA’s authority to restrict compounded semaglutide.
However, Judge Pittman ruled that the agency acted within its discretion, citing its evaluation of supply and demand data, including public remarks made by Novo Nordisk’s CEO about access challenges.
The FDA reportedly followed up with the company before finalizing its decision.
Under the ruling, larger outsourcing facilities were required to stop compounding semaglutide by May 22, while smaller pharmacies were ordered to cease operations by April.
This marks the second court defeat for compounding pharmacies contesting FDA actions on weight-loss drugs.
In May, Judge Pittman dismissed a similar challenge involving Eli Lilly’s Zepbound and Mounjaro, a case that is now under appeal.
The European Medicines Agency (EMA) has formally acknowledged a potential link between semaglutide, the key ingredient in Ozempic, Wegovy, and Rybelsus, and a rare but serious eye condition known as non-arteritic anterior ischemic optic neuropathy (NAION).
This disorder, which can result in vision loss, has now been added to the safety labels for these medications.
According to the EMA’s Pharmacovigilance Risk Assessment Committee, NAION will be listed as a rare side effect, meaning it may occur in up to 1 in 10,000 patients after a year or more of treatment.
This regulatory action marks the first official recognition of a connection between semaglutide use and the risk of NAION, following growing concern fueled by clinical research.
A major study from March 2025 involving 350,000 individuals found that patients taking Ozempic for two years faced more than double the risk of developing NAION compared to those using other type 2 diabetes treatments.
Despite the label change, Novo Nordisk maintains that its internal clinical trials and post-marketing surveillance data do not demonstrate a clear causal relationship between semaglutide and vision impairment.
Nonetheless, the pharmaceutical company has agreed to update its product labeling as requested by European regulators.
It continues to assert that the overall benefits of the medication outweigh the potential risks.
To date, the U.S. Food and Drug Administration (FDA) has not issued a statement regarding whether it will also investigate or act on these findings.
Emerging data suggest that GLP-1 receptor agonists—such as Ozempic and Wegovy—could affect fertility in both men and women, introducing new considerations for potential product liability litigation.
Registered dietitian Ayla Barmmer recently informed The New York Post that approximately 15% of her patients using GLP-1 medications have experienced fertility-related issues.
Within that group, 40% reported symptoms such as irregular menstrual cycles, delayed ovulation, or diminished sperm quality.
These reproductive disruptions appear to be linked to nutrient deficiencies brought on by rapid weight loss.
According to Barmmer, patients commonly exhibit deficiencies in vital nutrients including protein, vitamin B12, vitamin D, iron, calcium, and folate—all of which are crucial for reproductive function.
In women, these deficiencies may contribute to luteal phase defects, while men may see reduced testosterone levels and impaired sperm motility.
Additionally, clinicians warn that severe caloric restriction may signal the brain to suppress reproductive hormone production as a biological safeguard.
Paradoxically, GLP-1 drugs have also demonstrated benefits for fertility in certain populations.
For individuals with polycystic ovary syndrome (PCOS), improved insulin sensitivity and reduced inflammation—common effects of GLP-1 treatment—can enhance fertility outcomes.
This contradictory impact underscores the nuanced nature of GLP-1 drugs on reproductive health.
Should legal claims arise from infertility or adverse pregnancy outcomes, this scientific complexity may play a central role in determining liability and causation.
Barmmer advises that individuals planning to conceive should pursue tailored nutritional support to address any deficiencies.
She further recommends discontinuing GLP-1 medications eight to ten weeks prior to attempting conception, to help restore hormonal balance and improve reproductive readiness.
As research continues to unfold, the fertility implications of GLP-1 treatments are likely to remain a focal point in both clinical and legal contexts.
The Ozempic multidistrict litigation (MDL) added 73 new cases between May and June, raising the total to 1,882 lawsuits.
Most of these filings focus on gastroparesis, a serious gastrointestinal condition marked by prolonged vomiting, bloating, and frequent hospitalizations.
Plaintiffs allege that GLP-1 receptor agonists like Ozempic and Wegovy caused delayed gastric emptying, which has been documented in many cases through diagnostic imaging and gastroenterologist evaluations.
Lawyers are reviewing medical records to identify consistent patterns in drug usage, such as dosage escalation and combination with other GLP-1 medications.
The court is expected to establish initial discovery guidelines soon, which will shape the direction of the litigation moving forward.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
Health professionals are sounding the alarm over a growing trend dubbed “Ozempic teeth,” referring to a cluster of dental problems being reported by individuals taking semaglutide-based medications like Ozempic.
Patients have increasingly experienced issues such as tooth decay, gum irritation, enamel erosion, and persistent dry mouth while using GLP-1 drugs.
According to insights from Dr. Sandip Sachar and Dr. Meghan Garcia-Webb, these dental problems appear to stem from common side effects of the medication, including dehydration, acid reflux, and nausea-induced vomiting.
Although no clinical studies have yet established a direct link between these medications and oral health damage, many practitioners have observed a pattern.
Known effects of GLP-1 drugs—such as reduced thirst and slowed digestion—can suppress saliva production, a crucial defense against plaque buildup and acid erosion.
Without enough saliva, teeth are more susceptible to damage.
To help manage symptoms, doctors recommend adjusting the medication dosage or introducing additional treatments to control nausea.
Currently, dental complications are not recognized as part of ongoing Ozempic-related legal claims.
However, attorneys are monitoring emerging research and may pursue further legal action if stronger evidence ties oral health damage to GLP-1 medications.
The FDA is tightening enforcement on compounded versions of popular weight-loss drugs like Ozempic and Zepbound now that nationwide shortages have officially ended.
These compounded medications, custom-made by pharmacies, became a lower-cost alternative during the supply crunch—often priced around $350 compared to the $1,000 cost of the brand-name options.
With supply levels restored, the FDA is ordering compounding pharmacies to halt production of these off-brand versions.
Officials warn that compounded semaglutide products can pose safety risks due to inconsistent dosing, lack of standardized testing, and potential ingredient issues.
Some pharmacies have reportedly tried to bypass the crackdown by modifying dosages or ingredients, prompting additional concern from regulators.
Medical professionals are advising patients to stick with FDA-approved medications and consult their doctors before starting or continuing any compounded weight-loss treatments.
As the use of GLP-1 drugs like Ozempic, Wegovy, and Zepbound continues to rise, doctors are reporting a concerning side effect: “Ozempic feet.”
This term describes the discomfort and pain some users experience as a result of rapid weight loss, which reduces the natural fat padding on the soles of the feet.
The loss of cushioning can make walking painful, creating a sensation akin to stepping directly on bone.
Although not life-threatening, Ozempic feet can significantly impact mobility, making it difficult for individuals to stay active—something essential for long-term health and sustained weight management.
Medical experts caution that the pressure placed on bones and joints may lead to functional changes in gait, which can stress other areas of the body.
The issue may be especially troubling for patients with type 2 diabetes, who are already at higher risk for foot-related complications such as nerve damage and poor circulation.
As awareness of this side effect grows, more patients are coming forward to share how the drug has impacted their daily lives.
The Ozempic multidistrict litigation (MDL) continues to grow, with 124 new cases added over the past month.
Since January 1st, the total number of filings has increased by 478, reflecting a steady rise in claims.
This ongoing growth points to heightened awareness among patients who allege they suffered severe side effects after taking Ozempic and similar GLP-1 medications.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
A new lawsuit has been filed outside the GLP-1 multidistrict litigation (MDL), alleging that Ozempic caused severe vision loss.
On April 16, a North Carolina woman brought her case in federal court in New Jersey, claiming that she experienced blurred and diminished vision in her left eye after taking Ozempic.
Unlike most current lawsuits—consolidated in the Eastern District of Pennsylvania—that focus on gastrointestinal injuries like gastroparesis and intestinal obstruction, this case centers on vision damage.
The plaintiff alleges that Ozempic led to her diagnosis of non-arteritic anterior ischemic optic neuropathy (NAION), a rare and serious condition involving loss of blood flow to the optic nerve.
The lawsuit argues that Novo Nordisk failed to warn users about the risk of NAION.
This claim builds on research from Harvard published in July 2024, which found a significantly increased risk of NAION among semaglutide users, followed by additional studies in January 2025 and warnings from the Danish Medicines Agency.
The plaintiff used Ozempic from November 2023 to June 2024 and is now pursuing claims for failure to warn, negligence, breach of warranty, fraudulent concealment, and design defect. She seeks both compensatory and punitive damages.
While early bellwether trials in the GLP-1 MDL may shape outcomes for GI-related claims, this lawsuit remains distinct and may open the door for similar NAION cases in the future.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The U.S. Food and Drug Administration (FDA) has issued a public safety alert following the seizure of several hundred counterfeit Ozempic injections discovered within the U.S. market.
The fake products, labeled as 1-milligram Ozempic doses, were found to be circulating outside approved distribution channels. Patients and healthcare providers are urged to verify their prescriptions’ authenticity.
The counterfeit drugs were linked to lot number PAR0362 and featured serial numbers starting with 51746517.
These units were confiscated by the FDA on April 9, 2025, and an investigation is underway in collaboration with Novo Nordisk, the pharmaceutical company behind Ozempic.
While six adverse health incidents have been reported in connection to this batch, no direct link to the counterfeit medication has been confirmed as testing is still in progress.
This event highlights growing concerns about the popularity and misuse of Ozempic, especially as it’s increasingly sought after for off-label weight loss use.
It also follows a previous FDA alert from December 2023, when thousands of fake Ozempic units were intercepted.
Some of those earlier counterfeit products may still be in circulation.
It’s important to distinguish these counterfeit drugs from legally compounded versions, which are sometimes produced by pharmacies during supply shortages under specific regulatory guidelines.
However, in February 2025, the FDA confirmed that shortages of both Ozempic and its weight loss counterpart, Wegovy, had been resolved, reducing the need for compounded alternatives.
The FDA and Novo Nordisk continue to monitor the situation and are working to stop the spread of unauthorized versions of the drug.
Medical professionals are encouraged to report any suspected counterfeit products immediately.
Pfizer has officially discontinued development of its experimental weight loss pill, danuglipron, following a case of liver enzyme elevation in a clinical trial.
The incident has added to the growing scrutiny around GLP-1 receptor agonists—a drug class that includes well-known medications like Ozempic, Wegovy, and Mounjaro—amid the increasing demand for obesity treatments.
The trial participant did not exhibit symptoms typically associated with liver damage, but lab tests revealed heightened liver enzyme levels, which can signal potential liver injury.
These enzyme levels reportedly returned to baseline after the individual stopped taking the drug.
Pfizer noted that the incident took place during a study phase involving a rapid dosage increase and emphasized that the decision to halt danuglipron’s development came after a comprehensive review of clinical findings and regulatory input.
This is the second time Pfizer has pulled back on danuglipron, with the company previously discontinuing a twice-daily version in late 2023 due to tolerability concerns.
Though the newer, once-daily formulation had shown promise in meeting certain efficacy and safety benchmarks, Pfizer has now opted to end its pursuit entirely.
GLP-1 drugs, such as semaglutide (sold under brand names like Ozempic and Wegovy), act by mimicking hormones that help regulate appetite and blood sugar.
Initially developed for managing Type 2 diabetes, these drugs have surged in popularity thanks to their notable weight loss effects, often being prescribed off-label for obesity.
Pfizer acknowledged that the elevated liver enzymes observed in its trial were in line with rates seen in other approved GLP-1 medications.
Nonetheless, the broader safety profile of GLP-1 drugs remains under close watch, particularly as more individuals begin using them for extended periods.
Although danuglipron is no longer in the pipeline, Pfizer remains committed to obesity drug development.
The company is now focusing on other investigational treatments, including a new oral candidate targeting the GIPR pathway, which is currently undergoing phase two clinical trials.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
A recent study has found a rare but concerning connection between the use of Ozempic and severe drops in blood sugar levels, even when the medication is not combined with other treatments
The research, published on April 7 in the Annals of Internal Medicine, was carried out by the Centers for Disease Control and Prevention (CDC) in collaboration with Cambridge Health Alliance.
It looked at emergency room visits connected to semaglutide-based drugs like Ozempic and Wegovy.
Between 2022 and 2023, the study estimated that roughly 24,500 ER visits were related to these medications.
More than 20,000 of those occurred in 2023 alone. The majority of visits involved digestive issues—such as nausea, vomiting, and diarrhea—most often linked to incorrect dosing.
However, around 16% of the reported cases involved hypoglycemia, or low blood sugar.
This condition can be dangerous, potentially causing symptoms like fainting, seizures, or even stroke-like episodes.
Hypoglycemia is typically seen when medications like Ozempic are used together with insulin or other blood sugar-lowering drugs.
But this study noted a few instances where patients experienced severe low blood sugar while only using Ozempic.
The findings have raised questions, especially as the number of people using semaglutide medications continues to climb.
Another concern is the rising use of non-FDA-approved, compounded versions of semaglutide—often purchased online due to shortages of name-brand drugs.
Poison control centers have seen a major increase in calls related to these products, mostly due to dosing mistakes.
In some cases, patients accidentally took 10 times the intended dose because of confusing syringe measurements.
Since 2019, there has been a 1,500% spike in poison center reports involving GLP-1 medications like Ozempic, with 1,678 cases already reported in 2025.
These safety concerns may become a bigger part of legal evaluations as the use of these drugs expands.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The number of Ozempic Lawsuits continues to rise as more individuals report serious side effects linked to the drug.
In March, 1,521 total lawsuits had been filed.
By April 1, that number increased to 1,685, reflecting 164 new filings in just one month.
These lawsuits allege that Ozempic, a medication prescribed for type 2 diabetes and weight management, has caused severe gastrointestinal complications, pancreatitis, and other health risks.
Plaintiffs argue that Novo Nordisk failed to provide adequate warnings about these dangers, leaving patients unaware of the potential consequences.
As the litigation grows, courts will soon begin evaluating claims and preparing for early trials that could shape future settlement discussions.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
Plaintiffs in the GLP-1 drug litigation—including claims involving Ozempic, Wegovy, and Mounjaro—have asked a federal judge to reject dismissal efforts by Novo Nordisk and Eli Lilly.
In a March 18 filing, they argue that the drug makers exaggerated the benefits of GLP-1 medications while downplaying serious gastrointestinal risks, such as gastroparesis and intestinal blockages.
The multidistrict litigation (MDL), now encompassing over 1,500 cases, is being overseen by Judge Karen Marston in the Eastern District of Pennsylvania.
Plaintiffs claim the companies aggressively marketed these drugs while failing to warn about life-altering side effects.
Earlier this year, the defendants moved to dismiss most of the claims, arguing only the failure-to-warn claims should stand.
Plaintiffs counter that their complaint includes broader allegations, such as deceptive marketing, negligence, breach of warranty, and product design flaws.
The court must still determine whether federal law preempts these claims and if plaintiffs must show diagnostic proof of injury.
If the litigation moves forward, bellwether trials will help shape the outcome of future cases and potential settlements.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The Ozempic Lawsuit is ongoing.
Eli Lilly, the maker of Mounjaro and Zepbound, is urging the court to impose stricter diagnostic proof requirements in the ongoing GLP-1 gastroparesis litigation.
In a motion filed on March 5, the company argues that plaintiffs must present contemporaneous medical testing to verify a gastroparesis diagnosis, claiming clinical symptoms alone are unreliable.
Plaintiffs push back, asserting that physicians often rely on medical history and clinical observation to diagnose the condition.
The outcome of this motion could affect many of the 1,500+ cases pending in the federal multidistrict litigation.
The litigation is overseen by U.S. District Judge Karen Marston in Pennsylvania.
A March 18 status conference will address case progress, although no ruling is expected on the testing motion yet.
If the lawsuits advance, bellwether trials could begin in 2027, setting the stage for future Ozempic and GLP-1 settlements.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
Lawsuits continue to grow against the makers of GLP-1 medications like Ozempic, Mounjaro, and Wegovy, as patients report unexpected and irreversible vision problems.
One of the most serious conditions identified is non-arteritic anterior ischemic optic neuropathy (NAION), which causes sudden vision loss.
James Norris, a 56-year-old mechanic, is one of many who experienced vision deterioration after taking Mounjaro, despite seeing benefits in weight loss and diabetes control.
Recent studies published in 2024 show an increased risk of NAION, particularly in patients using these drugs for weight loss.
Despite the findings, manufacturers Novo Nordisk and Eli Lilly have not updated their warning labels to reflect this potential danger.
Plaintiffs allege the companies failed to disclose the risk and misled both patients and medical professionals.
Some, like Cheryl Bovee—now legally blind after taking Ozempic—are demanding accountability for the harm suffered.
With more patients stepping forward, the litigation is expected to expand, potentially leading to changes in how these medications are labeled and marketed.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
A federal judge has ruled against compounding pharmacies producing their own versions of Eli Lilly’s diabetes and weight loss drugs, Zepbound and Mounjaro.
Judge Mark Pittman of the Northern District of Texas issued the decision following a lawsuit filed by the Outsourcing Facilities Association (OFA) in October.
The OFA argued that the FDA’s removal of tirzepatide, the active ingredient in these drugs, from its shortage list would limit access to essential treatments and increase drug prices.
This ruling effectively bans the production of compounded versions of these medications, which were allowed during the shortage.
After the lawsuit, the FDA had temporarily paused enforcement but reaffirmed its position in December.
Smaller pharmacies had until February 18 to stop making compounded tirzepatide, with larger outsourcing facilities required to cease production by March 19.
The OFA has signaled an appeal, with similar litigation ongoing regarding semaglutide, the active ingredient in Ozempic.
Eli Lilly praised the decision, stating it would stop the production of unapproved and potentially unsafe versions of the drugs.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The FDA has officially declared the Ozempic and Wegovy shortages over after more than two years of supply chain disruptions.
Novo Nordisk has confirmed that production has stabilized, allowing the company to meet current and future demand in the U.S.
Despite this announcement, some patients may still experience temporary delays as shipments move through distribution channels.
Pharmacies are advising individuals to check availability before refilling prescriptions.
Additionally, compounding pharmacies will be required to phase out off-brand versions of semaglutide in the coming months.
While supply issues are resolving, lawsuits against Novo Nordisk continue to grow. Plaintiffs allege that the manufacturer failed to provide adequate warnings about the risks associated with Ozempic and other GLP-1 drugs, particularly severe gastrointestinal injuries such as gastroparesis, small bowel obstructions, and gallbladder disease.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
As of February 2025, the number of Ozempic lawsuit cases reached 1,443, marking an increase of 112 cases from the 1,331 filed in January 2025.
This rise in filings is linked to continuing concerns over Ozempic’s side effects, with plaintiffs alleging various health complications caused by the drug.
The growing number of cases reflects heightened awareness of the potential risks associated with Ozempic and a surge in legal actions from those impacted.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
A recent study has highlighted concerns about websites selling compounded versions of popular weight-loss medications, including Novo Nordisk’s Wegovy and Eli Lilly’s Zepbound, often without disclosing important safety information.
Researchers analyzed 79 websites promoting compounded GLP-1 receptor agonists, such as semaglutide and tirzepatide, and uncovered critical transparency issues:
- Over half did not disclose that their products were not FDA-approved.
- 37% falsely suggested FDA approval, while 14% failed to clarify they were offering compounded versions.
- Nearly 50% omitted vital details about side effects, precautions, and warnings.
- Approximately 40% exaggerated the drugs’ benefits, potentially misleading consumers.
Compounded drugs are typically produced during shortages of branded medications but do not undergo FDA verification for safety or effectiveness.
The persistent supply shortages of Wegovy and Zepbound have driven demand for these alternatives, sold at prices between $231 and $330 for the first month.
Outgoing FDA Commissioner Robert Califf and other experts have raised concerns about the safety of compounded drugs sold online, emphasizing that patients may not fully understand what they are purchasing.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The Ozempic Lawsuit is ongoing.
The Ozempic Lawsuit continues to evolve as the federal multidistrict litigation (MDL) over GLP-1 drugs, including Ozempic and Saxenda, expands.
While claims now encompass Saxenda, the MDL remains focused on gastrointestinal injuries such as gastroparesis, small bowel obstruction, and gallbladder complications.
A pivotal evidentiary hearing is scheduled for May 14, 2025, before U.S. District Judge Karen Marston.
During this hearing, the court will evaluate the scientific evidence presented by plaintiffs to determine if a causal link exists between GLP-1 drugs and the alleged injuries.
This determination of “general causation” is critical to the progression of the lawsuits.
Currently, the MDL includes over 1,300 lawsuits filed against manufacturers Novo Nordisk and Eli Lilly, with the number of cases expected to grow as more patients report complications such as stomach paralysis and intestinal blockages.
Experts predict the scope of the litigation could encompass tens of thousands of claims by the end of 2025, reflecting the widespread use of GLP-1 medications.
Key developments in the litigation include:
- Regular Status Conferences: To monitor the progression of cases.
- Fact Discovery on Causation: Set to conclude by July 2025.
- Trials: Expected to commence in late 2026 or early 2027.
The outcomes of these early trials will likely play a significant role in shaping future settlement discussions and the overall trajectory of the litigation.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The Ozempic Lawsuit centers on claims that the diabetes and weight loss medication Ozempic has led to severe gastrointestinal side effects, including gastroparesis, nausea, and vomiting.
Plaintiffs assert that the drug manufacturers, Novo Nordisk and Eli Lilly, failed to provide adequate warnings about these risks.
In December 2024, 1,300 cases were pending in the Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs) Products Liability Litigation.
By January 2025, this number rose to 1,331, with 31 new claims added.
Gastroparesis and related complications can severely impact quality of life, often necessitating ongoing medical treatment and intervention.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The FDA has expressed concerns about the use of unapproved GLP-1 drugs like semaglutide (Ozempic) and tirzepatide for weight loss.
These compounded and counterfeit versions bypass FDA safety reviews, creating serious risks for patients.
Reports indicate severe side effects such as nausea, vomiting, and even hospitalization due to dosing errors and improperly labeled products.
Compounded versions often include unauthorized ingredients, like salt forms of semaglutide, or exceed the approved dosage guidelines, which increases risks even further.
Counterfeit Ozempic and illegally marketed versions sold online or labeled “not for human consumption” are under scrutiny for containing harmful or inactive ingredients.
Patients are encouraged to use only FDA-approved drugs from licensed pharmacies and consult their healthcare provider for any concerns.
Adverse events can be reported to the FDA through its MedWatch program to help protect public health.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The Judicial Panel on Multidistrict Litigation (JPML) has recently denied a motion to expand the existing Weight Loss MDL, which currently includes drugs like Ozempic, to also cover claims related to blood clot injuries.
The plaintiffs had requested this expansion to centralize lawsuits concerning possible blood clot injuries allegedly tied to these medications.
Surprisingly, the defendants supported the motion, likely to prevent fragmented litigation in courts across the nation.
However, the JPML determined that incorporating all potential injury claims related to these popular weight loss drugs would make the MDL too complex and unmanageable both procedurally and substantively.
Consequently, blood clot injury claims will not be added to the MDL.
Plaintiffs with such claims now have two options: they can either initiate a separate MDL specifically for blood clot injuries or pursue individual lawsuits in various state or federal courts.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
Novo Nordisk’s Ozempic is facing scrutiny after two Danish studies found a potential link between the diabetes drug and an increased risk of non-arteritic anterior ischemic optic neuropathy (NAION), a rare condition causing vision loss due to reduced blood flow to the optic nerve.
The studies suggest that users of Ozempic may be more than twice as likely to develop NAION compared to those on other diabetes medications.
The Danish Medicines Agency has reported 19 cases of NAION among Ozempic users in Denmark, with a noticeable increase in overall cases since the drug’s market debut in 2018.
These findings have led Danish regulators to urge the European Union’s drug agency to review the data.
The studies, which analyzed data from hundreds of thousands of patients in Denmark and Norway, build on previous research from Harvard University.
While the absolute risk remains low—estimated at 0.3% to 0.5% over 20 years of use—further research is required to determine if similar risks exist for Wegovy, Ozempic’s counterpart for obesity treatment.
Novo Nordisk insists that the benefit-risk profile of semaglutide, the active ingredient in Ozempic, remains unchanged and stresses that patient safety is a priority.
Analysts believe the risk of NAION is unlikely to have a significant impact on prescriptions unless Ozempic is proven to be uniquely susceptible to this risk compared to other GLP-1 drugs.
Additional studies are anticipated to provide more clarity on the long-term safety profile of Ozempic and related medications.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The Ozempic and GLP-1 Weight Loss Drugs lawsuit addresses allegations that these medications have caused serious gastrointestinal side effects, such as gastroparesis, nausea, and vomiting.
Plaintiffs claim that manufacturers failed to sufficiently warn users about these risks.
In November, 1,221 cases were filed, increasing to 1,300 in December with 79 new claims.
This rise reflects growing awareness of the potential dangers linked to GLP-1 drugs like Ozempic.
Severe gastrointestinal issues can severely impact the quality of life, often requiring ongoing medical treatment for those affected.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The multidistrict litigation (MDL) for GLP-1 medications, including Ozempic, Wegovy, and Mounjaro, reached a significant milestone with the filing of a Master Complaint.
This consolidated filing presents common allegations against drug manufacturers Novo Nordisk and Eli Lilly, accusing them of failing to sufficiently warn about severe gastrointestinal side effects such as gastroparesis, intestinal obstruction, and ischemic bowel.
The litigation currently involves over 1,200 claims and is expected to grow as more individuals connect their injuries to these medications.
The Master Complaint includes a variety of claims, such as:
- Failure to Warn
- Fraudulent Concealment
- Negligent Design
- Strict Liability for Design Defects
- Wrongful Death and Loss of Consortium
Judge Karen S. Marston, who is overseeing the MDL in the Eastern District of Pennsylvania, is anticipated to approve a Short Form Complaint soon.
This will simplify the process for future plaintiffs, allowing them to adopt the Master Complaint’s allegations and add their specific details.
Moving forward, key issues will be addressed, including setting standards for diagnostic evidence and preparing for bellwether trials.
These trials, while not directly impacting other cases, may influence settlement negotiations in the expanding litigation.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The Ozempic Lawsuit is ongoing.
The Ozempic Lawsuit continues to grow, with claims asserting that the diabetes medication Ozempic has led to severe gastrointestinal side effects, including gastroparesis (stomach paralysis), nausea, and vomiting.
Plaintiffs allege that Novo Nordisk, the drug’s manufacturer, failed to adequately warn patients and healthcare providers of these risks.
In October, there were 1,090 cases filed in the Ozempic lawsuit, rising to 1,221 in November, with an additional 131 filings.
Gastroparesis, one of the primary side effects cited, can lead to chronic nausea, vomiting, and malnutrition, significantly affecting quality of life.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The Ozempic Lawsuit is ongoing.
The FDA is currently reevaluating its recent decision to remove Eli Lilly’s obesity and diabetes medications, Zepbound and Mounjaro, from the shortage list, allowing compounding pharmacies to continue producing their versions temporarily.
This reevaluation comes after the Outsourcing Facilities Association (OFA) filed a lawsuit, arguing that the FDA’s decision ignored evidence of ongoing supply shortages.
The lawsuit, filed in the U.S. District Court for the Northern District of Texas, claims that the FDA failed to follow federal procedures by not providing notice or allowing public comment before removing these drugs from the shortage list.
In response, the FDA has agreed to revisit its decision and permit compounders to continue manufacturing the drugs while discussions continue.
A status report from both parties is expected by November 21.
This legal battle underscores ongoing supply chain issues with popular GLP-1 medications like Zepbound, Mounjaro, and Novo Nordisk’s Wegovy, all of which have seen surging demand.
Despite efforts by Lilly and Novo to ramp up production, shortages remain, particularly for the initial “starter” doses of Wegovy, which is still listed as being in shortage by the FDA.
If you or a loved one experienced severe stomach paralysis, bowel obstruction, ileus, or other health issues after taking Ozempic, you may qualify to file an Ozempic Lawsuit.
Contact the Ozempic Lawyers at TruLaw for a free consultation, or use the chatbot on this page to determine if you qualify for a case instantly.
The Ozempic Lawsuit is ongoing.
The Ozempic Lawsuit focuses on claims that the diabetes and weight loss medication, Ozempic, has caused severe gastrointestinal issues, including gastroparesis (paralysis of the stomach), nausea, vomiting, and other serious side effects.
Plaintiffs allege that manufacturers Novo Nordisk and Eli Lilly failed to adequately warn patients and healthcare providers about the risks associated with Ozempic and other GLP-1 drugs.
In September, 869 cases were filed in the Ozempic MDL.
By October, the number of pending Ozempic Lawsuits had risen significantly to 1,090, an increase of 221 filings.
Ozempic, along with other GLP-1 weight loss medications, is primarily used to manage blood sugar levels in individuals with type 2 diabetes.
However, these drugs have been linked to severe digestive issues such as gastroparesis, a condition that delays stomach emptying, resulting in persistent nausea, vomiting, and even malnutrition.
If you or a loved one suffered from severe stomach paralysis, bowel obstruction, ileus, or other serious health issues after taking Ozempic or similar medications, you may be eligible to file a lawsuit against the drug manufacturers.
Contact the Ozempic Attorneys at TruLaw for a free consultation.
You can also use the chatbot on this page for a free case review to see if you qualify to file an Ozempic Lawsuit instantly.
The Ozempic Lawsuit is ongoing.
The multidistrict litigation (MDL) against pharmaceutical giants Novo Nordisk and Eli Lilly, involving GLP-1 drugs such as Ozempic, Wegovy, Trulicity, and Mounjaro, continues to progress as plaintiffs claim these medications have caused serious gastrointestinal injuries.
The primary allegation is that these drugs are linked to gastroparesis, a condition that paralyzes stomach muscles.
With over 900 complaints consolidated into this MDL, 85% of the cases cite gastroparesis as a primary injury.
Recently, both sides presented key scientific evidence during a “science day” session, a common event in large-scale pharmaceutical lawsuits.
This session allowed attorneys and experts to discuss critical data regarding the drugs, their effects, and the underlying medical science.
Central to the plaintiffs’ argument is the claim that these medications cause gastroparesis, while the defense contends that the drugs are designed to delay gastric emptying, which they argue is distinct from gastroparesis.
This technical difference is a key point in the defense’s argument.
Despite these defenses, plaintiffs’ attorneys emphasize the real-life impact of gastroparesis on their clients, which they believe will be more compelling in court.
No trial date has been set yet, but the “science day” laid essential groundwork for the future of this litigation.
If you or a loved one suffered from severe stomach paralysis, bowel obstruction, ileus, or other health issues after taking Ozempic or other GLP-1 drugs, you may be eligible to file an Ozempic Lawsuit against the drug manufacturers.
Contact the Ozempic Attorneys at TruLaw for a free consultation.
You can also use the chatbot on this page for a free case review to find out if you qualify to file an Ozempic Lawsuit instantly.
The Ozempic Lawsuit is ongoing.
A recent report highlights that Ozempic and other weight loss drugs, including Wegovy, have been linked to 162 deaths in the U.S. over the past six years, according to data from the FDA Adverse Event Reporting System (FAERS).
Although these deaths haven’t been definitively proven to be caused by semaglutide, the active ingredient in these drugs, they were mentioned as factors in the fatalities.
The FAERS database has recorded 62,000 adverse reactions to these drugs, with 10,000 classified as serious.
Despite the growing concerns and reports of side effects, including gastrointestinal issues, pancreas inflammation, and gallbladder problems, the drugs continue to be widely used for weight loss.
Furthermore, in the MDL involving Ozempic and other GLP-1 medications, the U.S. District Court is evaluating whether plaintiffs must provide specific diagnostic testing to prove that they developed gastroparesis.
Specific diagnostic testing refers to a medical process where precise tests, such as gastric emptying studies, are used to confirm whether a patient has a particular condition, such as gastroparesis, by objectively measuring how well the stomach empties food into the intestines.
Additionally, the court will consider if the claims are preempted by federal law and determine whether GLP-1 drugs like Ozempic can cause the injuries in question, with decisions expected to impact the progression and potential settlement of these lawsuits.
“Preempted by federal law” means that federal regulations take precedence over state laws, preventing states from enforcing their own laws if they conflict with federal rules.
If you or a loved one suffered from severe stomach paralysis, bowel obstruction, ileus, or other health issues after taking Ozempic, you may be eligible to file an Ozempic Lawsuit against the drug makers.
Contact the Ozempic Attorneys at TorHoerman Law for a free consultation.
You can also use the chatbot on this page for a free case review to find out if you qualify to file an Ozempic Lawsuit instantly.
The Ozempic weight loss litigation is advancing, with Science Day scheduled to take place soon.
Science Day is a key hearing in mass tort cases where both sides present evidence, typically including scientific studies, key documents, and expert testimonies.
The hearing’s purpose is to provide the Court with a comprehensive understanding of the product and the main issues at hand.
Each judge approaches Science Day differently, but it’s usually one of the first opportunities for the Court to dive into the details of the case.
The hearing typically lasts several hours, featuring presentations and open dialogue in a question-and-answer format.
For the Ozempic litigation, Science Day will give all parties a clearer view of the upcoming legal proceedings.
After Science Day, Judge Marston has scheduled monthly Case Management Conferences in September, October, November, and December, indicating that this litigation is expected to pick up speed as the year progresses
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The number of Ozempic lawsuits has grown significantly, increasing from 346 in August to 869 in September.
Ozempic, which contains semaglutide as its active ingredient, has been associated with serious gastrointestinal problems, including gastroparesis (delayed stomach emptying) and pancreatitis.
Additionally, some users have reported thyroid tumors and other severe health complications.
These risks have prompted numerous lawsuits against Novo Nordisk, with allegations that the company failed to provide sufficient warnings about these potential dangers.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The Ozempic Lawsuit is ongoing, and our lawyers are accepting new clients.
Gastroparesis is a condition in which the stomach empties its contents into the small intestine more slowly than normal.
Common symptoms include nausea, vomiting, bloating, feeling full quickly, and abdominal pain.
Potential causes include diabetes, surgeries, infections, and certain medications.
Common causes of gastroparesis:
- Idiopathic: Accounts for about 50% of cases with no identifiable cause.
- Diabetes: More common in type 1 diabetes but also occurs in type 2.
- Post-surgical: Often occurs after surgeries impacting the stomach or vagal nerve.
- Postinfectious: Usually self-limiting but can sometimes lead to chronic issues.
How Does Ozempic Work?
Ozempic (semaglutide) is a medication primarily used for managing type 2 diabetes.
It functions by mimicking a hormone called GLP-1, which regulates blood sugar levels.
Ozempic boosts insulin production and slows the rate at which food leaves the stomach, aiding in blood sugar stabilization.
This slowing effect can also promote weight loss by prolonging the sensation of fullness.
Link Between Ozempic and Gastroparesis
Ozempic slows stomach emptying to help manage blood sugar, but this can worsen or trigger gastroparesis in some individuals.
This side effect has resulted in lawsuits against Novo Nordisk for allegedly not providing adequate warnings to users.
As a GLP-1 agonist, Ozempic mimics a hormone that delays food movement through the digestive tract, leading to symptoms like nausea and vomiting.
For those already prone to delayed gastric emptying, Ozempic can exacerbate the condition.
Numerous lawsuits argue that Novo Nordisk should have better communicated the risks of severe gastrointestinal side effects, including gastroparesis.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
The Ozempic Lawsuit is ongoing, and our lawyers are accepting new clients.
The lawsuit centers on claims against Novo Nordisk, the manufacturer of Ozempic, a popular medication used for the treatment of type 2 diabetes.
Plaintiffs allege that Ozempic causes severe gastrointestinal issues, including gastroparesis, which can lead to debilitating symptoms such as nausea, vomiting, and severe abdominal pain.
These side effects have prompted numerous individuals to file lawsuits, arguing that Novo Nordisk failed to adequately warn users of these potential risks.
According to recent filings, the number of cases related to the Ozempic Lawsuit has been steadily increasing.
As of August 1st, there are 346 cases pending, the same amount as July.
The lawsuits argue that Novo Nordisk was aware, or should have been aware, of the potential for these severe side effects but failed to provide sufficient warnings to consumers and healthcare professionals.
If you or a loved one suffered from severe stomach paralysis, bowel obstruction, ileus, or other health issues after taking Ozempic, you may be eligible to file an Ozempic Lawsuit against the drug makers.
Contact the Ozempic Attorneys at TruLaw for a free consultation.
You can also use the chatbot on this page for a free case review to find out if you qualify to file an Ozempic Lawsuit instantly.
A recent study published in JAMA Ophthalmology indicates that patients using Ozempic and Wegovy may have a higher risk of developing non-arteritic anterior ischemic optic neuropathy (NAION), a rare condition leading to sudden vision loss.
Key findings include:
- Diabetic patients on Semaglutide medications are over four times more likely to develop NAION.
- Overweight or obese individuals using these drugs face a sevenfold increase in risk.
- Approximately 100 cases were identified annually over six years, with the highest risk observed within the first year of medication use.
Novo Nordisk, the manufacturer of Ozempic and Wegovy, acknowledges the study but maintains that the data does not establish causation.
Despite the potential risk, NAION remains relatively uncommon compared to the benefits provided by these medications.
The U.S. Food and Drug Administration (FDA) lists vision changes among the possible side effects of semaglutide.
Novo Nordisk is conducting ongoing trials to explore the link between semaglutide use and diabetic retinopathy, with results expected by 2027.
Experts recommend that patients taking semaglutide or considering treatment discuss the risks and benefits with their doctors, particularly those with other optic nerve issues such as glaucoma or preexisting visual loss.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
A recent study from the University of Copenhagen raises new concerns about GLP-1 medications, including Ozempic, Wegovy, and Mounjaro, highlighting potential bone density loss.
Published in JAMA Network Open, the study found that using these medications without combining them with exercise can lead to a decrease in bone mineral density (BMD) in critical areas like the hips and spine.
Ozempic, initially approved for Type 2 diabetes in 2017, has gained popularity as a weight loss drug. Its active ingredient, semaglutide, is also present in Wegovy, a higher-dose version specifically for weight loss.
Despite aggressive marketing promoting these drugs as safe and effective, GLP-1 medications have been linked to severe health risks, including gastroparesis—a condition where the stomach is paralyzed, leading to long-term gastrointestinal issues.
Thousands of lawsuits have been filed against the manufacturers for allegedly failing to warn about these risks, prioritizing profits over consumer safety.
The latest study by Dr. Simon Birk Jensen and colleagues involved a randomized clinical trial with 195 participants.
The findings revealed that participants who combined GLP-1 treatment with exercise achieved the most significant weight loss while maintaining bone health.
Those who used Victoza alone experienced a decrease in BMD.
The study showed the necessity of exercise to mitigate the decrease in bone mineral density associated with GLP-1 medications.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
Recent research highlights a significant concern for users of glucagon-like peptide-1 receptor agonists (GLP-1RAs), commonly prescribed for diabetes management.
A study published in Gastroenterology on March 27, 2024, indicates an increased risk of aspiration pneumonia associated with GLP-1RA use during endoscopic procedures involving propofol sedation targeting the upper gastrointestinal tract.
The retrospective cohort study analyzed health records from 80 healthcare organizations, involving adults aged 21 to 70 who underwent upper and lower endoscopies between 2018 and 2020.
Results show that GLP-1RA users, defined as individuals with a history of use exceeding six months and with at least two refills within six months prior to the procedure, experienced a higher incidence rate of aspiration pneumonia (0.83%) compared to nonusers (0.63%), corresponding to a hazard ratio of 1.33.
This risk was notably higher in procedures involving propofol sedation, specifically upper GI endoscopies, with no significant risk noted in lower GI procedures.
Dr. Ali Rezaie, Medical Director of Gastroenterology Motility at Cedars-Sinai Medical Center, emphasized the need for context, noting that while the relative risk increases by 33%, the absolute risk remains low at 0.2%.
Conversely, the American Gastroenterological Association has not found evidence to support this precaution in their latest clinical update.
Despite this, some medical centers, including Cedars-Sinai, have begun advising patients to discontinue GLP-1RA use one week prior to elective procedures.
Further guidance is expected as new recommendations are being developed, reflecting a cautious approach towards managing the increased risk of aspiration in GLP-1RA users.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
You can also use the chatbot on this page for an instant case evaluation.
A mass tort lawsuit is currently underway in a federal court in Philadelphia, addressing serious concerns regarding the safety and regulatory oversight of widely used weight-loss medications including Ozempic, Wegovy, Rybelsus, Trulicity, and Mounjaro.
These drugs, primarily known as GLP-1 agonists, were initially approved for managing Type 2 diabetes but have gained popularity for weight loss purposes.
Recent data reveals a significant surge in their usage, with over 15 million Americans using these medications as of last month, marking a substantial increase in prescriptions by 40 times since 2018.
The core of the lawsuit alleges that Novo Nordisk and Eli Lilly, the manufacturers of these drugs, failed to provide sufficient warnings about potential severe gastrointestinal side effects.
Reported adverse effects include gastroparesis, intestinal obstructions, and pancreatitis, concerns that have been substantiated by recent studies and warnings from the Food and Drug Administration (FDA).
Legal experts indicate that this case not only scrutinizes the drug manufacturers but also casts doubt on the FDA’s approval and monitoring processes.
The ramifications of this lawsuit could extend beyond the courtroom, potentially leading to stricter regulatory measures for GLP-1 agonists and impacting their future market availability and consumer trust.
Amidst growing endorsements from celebrities and increasing awareness of their health benefits, these weight-loss drugs have experienced a surge in demand.
However, the ongoing lawsuit and the negative publicity surrounding it could temper future demand.
he outcome of this litigation holds significant implications for drug safety perceptions and the broader regulatory landscape of pharmaceuticals.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
You can also use the chatbot on this page for an instant case evaluation.
The Ozempic lawsuit is growing as more consumers become aware of GLP-1 side effects.
The U.S. Judicial Panel on Multidistrict Litigation (JPML) has recently transferred the combined litigation concerning Ozempic, Wegovy, and Mounjaro to U.S. District Judge Karen Marston in the Eastern District of Pennsylvania, following the unexpected passing of U.S. District Judge Gene E.K. Pratter.
Under the oversight of Judge Marston, the case now encompasses over 10,000 personal injury claims.
This litigation centers on serious claims against pharmaceutical giants Novo Nordisk and Eli Lilly and Co., alleging that they failed to adequately warn users about potential severe digestive side effects associated with these GLP-1 class drugs, which are commonly prescribed for diabetes management and weight loss.
Plaintiffs have linked these medications to severe health issues such as gastroparesis, intense vomiting, and bowel obstruction.
Significant legal proceedings include the completion of the plaintiff’s fact sheet, which will outline each claimant’s injuries, medical conditions, and specific drug usage.
While defense attorneys see this phase as a chance to potentially reduce the case’s breadth by dismissing claims related to counterfeit products or those without proper medical diagnoses, plaintiffs’ lawyers are contesting any premature dismissals.
They advocate for further discovery and the initiation of bellwether trials to comprehensively address and resolve the allegations.
Given the substantial public attention and the extensive use of these medications—reported to be used by one in eight U.S. adults—Judge Marston is expected to move forward with these proceedings.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
You can also use the chatbot on this page for an instant case evaluation.
The Ozempic lawsuit is growing as more consumers become aware of GLP-1 side effects.
The Ozempic lawsuit continues to gain attention as increasing numbers of consumers report severe side effects from GLP-1 receptor agonists, including Ozempic and Wegovy, which doctors frequently prescribe for managing obesity and diabetes.
Concerns are escalating about the risk of gastroparesis, a condition commonly known as stomach paralysis, which is often associated with these medications.
Gastroparesis slows down gastric emptying, leading to significant discomfort and complicating the management of metabolic diseases.
Recent studies highlight the risk of developing gastroparesis from GLP-1 medication use:
- Research unveiled at Digestive Week 2024 indicates a rise in gastroparesis occurrences in patients using GLP-1 drugs compared to those not taking these medications.
- A University of Kansas study, which reviewed health records of nearly 150,000 patients, found a 66% increased diagnosis rate of gastroparesis among individuals treated with GLP-1s.
- Further research by University Hospitals in Cleveland, examining over 336,000 individuals, discovered that the likelihood of developing gastroparesis increases after six months of GLP-1 therapy.
Although the overall risk is less than 1%, the data consistently show a significant relative increase.
As the popularity of Ozempic and Wegovy persists in diabetes and obesity management, ongoing investigations into their side effects, such as gastroparesis, are essential for patient safety.
Our Ozempic lawyers are actively investigating cases involving stomach paralysis and other severe side effects from these medications.
If you or a loved one used Ozempic, Wegovy, or compounded versions of the drugs, and subsequently developed gallbladder disease, stomach paralysis (gastroparesis), cyclic vomiting syndrome, or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
Awareness surrounding Ozempic and other GLP-1 drugs continues to grow.
The Ozempic multidistrict litigation is facing an unexpected complication due to the abrupt and tragic passing of U.S. District Judge Gene E.K. Pratter on May 17, 2024.
Judge Pratter, who was presiding over the Ozempic MDL in the Eastern District of Pennsylvania, passed away at the age of 75.
Judge Pratter’s death may have an impact on the ongoing Ozempic Lawsuits, which allege severe gastrointestinal side effects from glucagon-like peptide-1 (GLP-1) receptor agonists used in diabetes and weight loss treatments.
Some estimates indicate that nearly 2% of the U.S. population has been prescribed one of the GLP-1 medications, either for diabetes treatment or weight loss, it was widely expected that the litigation would become a major mass tort in the coming months.
These lawsuits claim that the drug manufacturers, including Novo Nordisk and Eli Lilly, failed to adequately warn users about the risks of medications such as Ozempic, Wegovy, and Mounjaro.
At the time of her passing, Judge Pratter was managing the initial stages of the MDL, which had been formed in February 2024, the MDL included 87 active lawsuits as of May 1, with expectations that the number could grow to several thousand due to the widespread use of these medications.
The reassignment of these cases to a new judge is now necessary, a process that will involve the review of existing records and a thorough understanding of the scientific and medical evidence, which could delay the proceedings.
A planned “Ozempic Science Day” on June 14, intended to clarify the scientific claims of the litigation, is now uncertain.
As the judicial system manages this transition, the continuity of the Ozempic MDL will be a priority.
If you or a loved one has suffered injuries from Ozempic or other GLP-1 drugs, you may be eligible to file a claim.
Contact us for a free consultation, or use the chatbot on this page to find out if you qualify for the Weight Loss Drugs Lawsuit.
Awareness surrounding Ozempic and other GLP-1 drugs continues to grow.
Growing concerns have surfaced regarding the serious side effects of Ozempic, a popular weight loss medication.
Medical professionals and patients report a range of adverse reactions linked to this drug and other GLP-1 medications.
A detailed examination reveals more than 9 million prescriptions of Ozempic were issued in the fourth quarter of 2022 alone.
Despite the high prescription rates, the number of users could be substantially higher, as many individuals obtain the drug without proper medical oversight due to insurance constraints.
Our Ozempic lawyers are closely monitoring the situation, as patients report severe side effects such as gastroparesis (stomach paralysis), gallbladder disease, cyclic vomiting syndrome, and more.
If you or a loved one has suffered injuries from Ozempic or other GLP-1 drugs, you may be eligible to file a claim.
Contact us for a free consultation, or use the chatbot on this page to find out if you qualify for the Weight Loss Drugs Lawsuit.
The Ozempic Lawsuit is progressing through the early stages in multidistrict litigation.
On May 9, 2024, U.S. District Judge Gene Pratter of Philadelphia officially appointed four attorneys as lead counsel in the multidistrict litigation (MDL) against diet drug manufacturers Novo Nordisk and Eli Lilly.
The legal proceedings have garnered attention not only for the nature of the claims but also for the nature of the claims but also for the innovative approach to selecting lead counsel, which could set a precedent for future large-scale litigations.
Judge Pratter’s method involves a combination of self-selection by a state of proposal lead counsels and confirmation through consensus among other plaintiffs’ lawyers.
The process led by Judge Pratter aims to streamline the complex management of thousands of cases in the Ozempic MDL.
As the Ozempic cases progress, the spotlight remains on the safety profile of these drugs.
Plaintiffs allege that side effects were not fully disclosed, impacting patient health when used as prescribed for diabetes management and weight loss.
Novo Nordisk has defended the safety of Ozempic by stating that all potential side effects are disclosed as per regulatory requirements.
If you or a loved one have suffered from stomach paralysis or other serious side effects from Ozempic, Wegovy, or other GLP-1 drugs, you may be eligible to file a claim.
Contact us for a free consultation, or use the chatbot on this page to find out if you qualify for an Ozempic Lawsuit.
The litigation surrounding Ozempic and other weight loss drugs is currently in the preliminary phase, and the number of lawsuits is expected to grow as the court sets the guidelines for filing cases.
A growing number of individuals are initiating legal actions against pharmaceutical leaders Eli Lilly and Novo Nordisk.
These firms manufacture a category of drugs known as GLP-1 agonists, which encompasses popular diabetes and weight loss medications like Ozempic, Wegovy, Rybelsus, Trulicity, and Mounjaro.
The plaintiffs allege that they experienced severe digestive complications, including the necessity for gallbladder removal and the development of gastroparesis, due to using these drugs.
U.S. District Judge Gene E. K. Pratter in Philadelphia is overseeing the consolidation of these legal actions because of the similar complaints regarding the adverse effects of these medications.
A significant portion of these legal challenges is directed at Novo Nordisk, the producer of Ozempic and Wegovy, with the potential for the total number of cases to reach into the thousands as more affected individuals step forward.
Novo Nordisk asserts the safety and effectiveness of its GLP-1 drugs, pointing to their 13-year presence on the market and thorough safety assessments conducted in partnership with the U.S. Food and Drug Administration (FDA).
This legal battle underscores the enormous popularity of GLP-1 drugs in the United States, utilized for both diabetes treatment and weight management.
With projections indicating 30 million users by 2030 and revenues exceeding $1 billion, the implications are significant for both the pharmaceutical sector and those claiming injuries from these drugs.
If you or someone you know has suffered from gastroparesis or other health issues after using Ozempic or similar GLP-1 medications, you might qualify for a weight loss drug lawsuit.
Reach out to TruLaw for a free, no obligation consultation.
For an immediate case evaluation, utilize the chatbot available on this webpage.
The Ozempic Lawsuit is developing, with legal actions focusing on claims related to severe gastrointestinal injuries attributed to the use of Ozempic and similar GLP-1 receptor agonist drugs.
As of early 2024, a federal panel has consolidated at least 55 lawsuits into a multidistrict litigation (MDL) in the Eastern District of Pennsylvania.
Ozempic Lawsuits allege that manufacturers Novo Nordisk and Eli Lilly, which also produces GLP-1 drugs, failed to adequately warn users about the potential for severe side effects such as gastroparesis (a condition causing delayed stomach emptying), intestinal obstruction, and other gastrointestinal injuries associated with these medications.
The consolidation into an MDL is aimed at streamlining the proceedings and ensuring consistent rulings across all cases.
The plaintiffs argue that despite the inclusion of warnings on the drug labels, the companies downplayed the severity of gastrointestinal issues.
The MDL includes claims related to a variety of drugs, including Novo Nordisk’s Ozempic, Wegovy, Rybelsus, and Eli Lilly’s Trulicity and Mounjaro.
Ozempic Lawyers anticipate that the number of lawsuits could grow significantly as part of the MDL.
A Louisiana federal judge largely rejected Novo Nordisk’s bid to dismiss the lawsuit, allowing the case to move forward, particularly on claims related to failure to warn about the risk of gastroparesis.
Ozempic Lawsuits and legal actions regarding other GLP-1 drugs highlight the importance of patients being fully informed about the potential side effects of weight loss medications, including those that could have severe implications for their health.
The consolidation of Ozempic Lawsuits into multidistrict litigation underscores the growing concern and legal scrutiny over the alleged severe side effects of Ozempic, Mounjaro, and other similar drugs.
If you or a loved one have suffered injuries after taking Ozempic or other similar drugs, you may be eligible to file an Ozempic Lawsuit.
Contact us for more information or use the chatbot on this page to find out if you qualify for the Ozempic Lawsuit instantly.
Lawsuits against the makers of Ozempic and other similar drugs are growing, and the highly popularized medication is continuing to make news headlines for the wrong reasons.
A 60-year-old Illinois resident claims that her use of Ozempic resulted in a blocked bowel and violent vomiting, leading to a torn esophagus which required a week-long hospitalization.
Ozempic, a weekly injection that helps lower blood sugar by helping the pancreas make more insulin, is also used to help mitigate weight gain and can help with weight loss.
This case signals a significant moment in the growing controversy over the blockbuster weight loss drug’s alleged severe side effects.
The injured Illinoisan’s case joins nearly 60 others alleging that Ozempic and Wegovy, another Novo Nordisk product, caused stomach paralysis among other debilitating conditions and side effects.
With a centralization of lawsuits in a Pennsylvania federal court, pharmaceutical companies face a troubling legal challenge.
As of February 5, 2024, the legal proceedings concerning Ozempic continue, and attorneys are still open to taking on additional cases.
The latest update from the Judicial Panel on Multidistrict Litigation (JPML) indicates that there are currently seven Ozempic-related lawsuits grouped in the recently established Multidistrict Litigation (MDL).
In the U.S., MDLs are specialized judicial procedures used to efficiently manage multiple civil lawsuits that share common factors, such as similar legal questions, facts, or defendants.
This approach is particularly useful when various parties file lawsuits concerning shared issues, like the health concerns associated with Ozempic and other drugs.
These related lawsuits are combined into one federal district court for the pretrial phase.
The primary objective of an MDL is to streamline legal proceedings.
The Ozempic Lawsuit is still ongoing.
Ozempic and Wegovy Lawsuit Investigation
New reports have indicated that a number of patients who used Ozempic and Wegovy, or compounded versions of these drugs, for weight loss have experienced serious side effects.
Stomach paralysis, a condition that affects nerves and muscles in the stomach, has been reported in multiple users of semaglutide products, specifically Ozempic and Wegovy.
Legal action is being investigated for users of Ozempic and Wegovy for these serious side effects.

If you or a loved one used Wegovy, Ozempic, or compounded versions of the drug for weight loss and subsequently developed gastroparesis, you may be eligible to file a legal claim.
Contact TruLaw for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
Our law firm is committed to helping people injured at no fault of their own seek compensation for what they’ve experienced.
Visit this page for further updates as our attorneys investigate this potential litigation.
FDA Warnings on Ozempic and Wegovy
The FDA has not yet produced warnings on the use of brand-name Ozempic and Wegovy for serious side effects, but the agency has released a warning on the use of compounded versions of the drug.
The FDA has received reports of adverse events related to compounded semaglutide products, claiming compounding pharmacies may be using salt forms of semaglutide, which are different active ingredients from those used in the FDA approved versions of Ozempic.

In addition to the FDA’s safety information page on Ozempic for weight loss, the FDA wrote to the National Association of Boards of Pharmacy about the agency’s concerns regarding the use of semaglutide salts in compounded versions of the drug.
What is the Controversy with Ozempic?
Ozempic is intended to be prescribed to diabetes patients.
Patients are required to meet medical criteria in order to obtain a valid Ozempic prescription.
Wellness clinics, compounding pharmacies, medical spas, and more have been prescribing Ozempic and compounded versions of the weight loss drug to people who do not meet criteria for prescriptions, potentially putting them at risk for serious side effects.
Novo Nordisk has even filed lawsuits against medical spas, wellness clinics, and weight loss clinics for selling illegally compounded versions.
Accusations in the lawsuit include false advertising, unfair competition, and trademark infringement.
There has been considerable media attention on Ozempic’s ability to help people shed weight in short amounts of time.
The New Yorker Magazine shed light on Ozempic’s rise in popularity among celebrities and influencers, and highlighted the drug’s potential to revolutionize how obesity and diabetes are treated.
What is Ozempic?
Ozempic is a popular diabetes drug that has been touted as a “miracle medication” used to maintain weight loss and control diets.
Ozempic (generic name: semaglutide) belongs to a class of drugs called glucagon-like peptide-1 (GLP-1) receptor agonists.
Initially approved by the U.S. Food and Drug Administration (FDA) in 2017, Ozempic was primarily designed for the treatment of type 2 diabetes.
Injected weekly, its main purpose is to improve blood sugar control in adults with type 2 diabetes, helping them manage their glucose levels and reduce the risk of diabetes-related complications.

GLP-1 receptor agonists, like Ozempic, work by mimicking the action of GLP-1 hormones, which naturally occur in the body and regulate insulin release in response to food intake.
By activating the GLP-1 receptors, Ozempic promotes insulin production, suppresses glucagon secretion (a hormone that raises blood sugar levels), slows down gastric emptying, and reduces appetite.
These actions contribute to better glycemic control in individuals with type 2 diabetes.
Ozempic and Weight Loss
Beyond its intended use for diabetes management, Ozempic has garnered attention for its potential in aiding weight loss.
Ozempic has been widely popularized by celebrities, influencers and social media trends, with Variety Magazine dubbing the drug:
“The worst kept secret in Hollywood.”
Produced by Danish pharmaceutical company Novo Nordisk, Ozempic

During clinical trials, some patients treated with Ozempic experienced significant weight loss compared to those receiving a placebo or other diabetes medications.
The exact mechanisms through which Ozempic induces weight loss are not fully understood, but it is believed to be a combination of reduced appetite, delayed gastric emptying, and potential effects on the brain’s reward system, leading to lower food intake and increased feelings of satiety.
Ozempic Side Effects
Ozempic has been linked to a number of side effects in patients.

These side effects include, but are not limited to:
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Abdominal pain
- Loss of appetite
- Headache
- Fatigue
- Dizziness
- Hypoglycemia (low blood sugar) when used in combination with other diabetes medications
- Injection site reactions (redness, swelling, or itching at the injection site)
- Pancreatitis (inflammation of the pancreas)
- Gallbladder problems (including gallstones)
- Kidney problems
- Thyroid tumors in animal studies (the relevance to humans is uncertain)
- Allergic reactions (rare but possible, may include rash, itching, swelling, severe dizziness, or trouble breathing)
- Gastroparesis (delayed emptying of the stomach)
Ozempic Stomach Paralysis (Gastroparesis)
One significant concern is the risk of gastroparesis, a condition characterized by delayed emptying of the stomach.
Gastroparesis occurs because Ozempic, like other GLP-1 receptor agonists, slows down the rate at which the stomach empties its contents.
While this can be beneficial for diabetes management and weight loss, it can also lead to complications in some individuals.

Symptoms of gastroparesis may include:
- Nausea
- Vomiting
- Bloating
- Feelings of fullness after only eating small amounts of food
- Persistent stomach pain
- Pain in the upper abdomen
- Heartburn
In severe cases, gastroparesis can lead to malnutrition and other digestive issues.
It is crucial for individuals prescribed Ozempic to be aware of these potential side effects and report any symptoms of gastroparesis or other adverse reactions to their healthcare provider promptly.
Patients should work closely with their healthcare team to monitor their response to the medication and adjust the dosage if necessary.
Ozempic and Gallbladder Disease
Scientific evidence has linked Ozempic usage and gallbladder disease, particularly gallstones.
An initial meta-analysis indicated an elevated risk of gallbladder disease with Ozempic use.
A 2022 Research Letter, based on FDA data, provided conclusive evidence showing that Ozempic users faced a significantly increased risk of both gallstones and acute gallbladder disease.
The risk was found to be higher at higher doses, for prolonged periods, and when Ozempic was used for weight loss.
Gallbladder disease encompasses various conditions affecting the gallbladder, including gallstones (cholelithiasis), inflammation (cholecystitis), and cancer.
Cholecystitis, caused by blockage of the gallbladder’s exit tube, is a serious condition requiring immediate treatment to prevent potentially fatal complications, and the most common treatment is gallbladder removal.
Gallstones are solid deposits that form inside the gallbladder and can vary in size, categorized as cholesterol stones (more common) or pigment stones, formed from bilirubin, a byproduct of liver red blood cell breakdown.
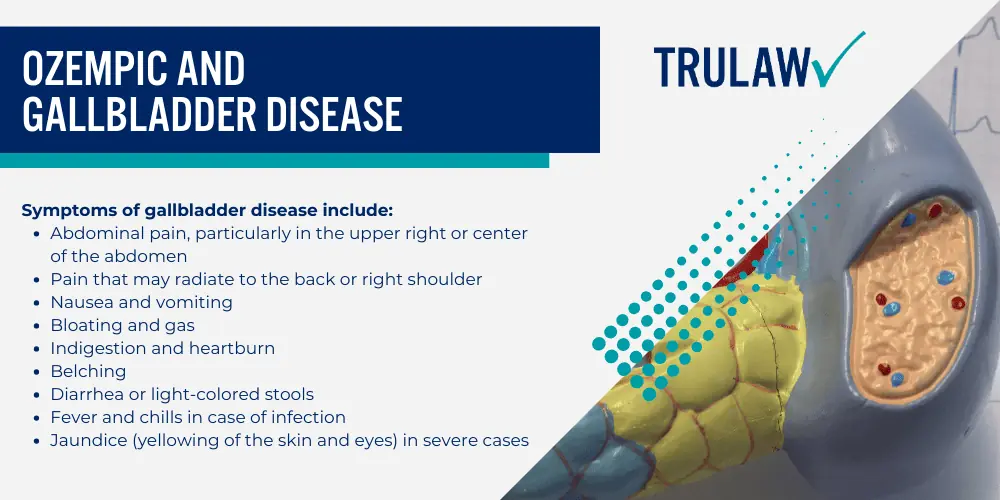
Symptoms of gallbladder disease include:
- Abdominal pain, particularly in the upper right or center of the abdomen
- Pain that may radiate to the back or right shoulder
- Nausea and vomiting
- Bloating and gas
- Indigestion and heartburn
- Belching
- Diarrhea or light-colored stools
- Fever and chills in case of infection
- Jaundice (yellowing of the skin and eyes) in severe cases
Before April 2022, the Ozempic warning label did not include gallbladder disease as a potential side effect.
Ozempic Use and Surgery
Anesthesiologists have raised concerns about the hazards of stomach paralysis in patients taking GLP-1 agonist medications like Ozempic before surgery, as there’s little information about the risks associated with stomach slowdown on these drugs.

Stomach contents suctioned from a patient on a GLP-1 agonist showed the stomach was full despite following pre-surgery fasting instructions, leading to a risk of aspiration into the lungs, which can have severe consequences.
The American Society of Anesthesiologists recommends patients stop taking these medications one week before surgery, but the appropriate duration of fasting or discontinuation is not yet fully understood due to limited scientific evidence.
What is Wegovy?
Wegovy is a prescription medication designed to combat obesity and promote weight loss.
Approved by the U.S. Food and Drug Administration (FDA), Wegovy is a higher-dose formulation of semaglutide, which was initially used for managing type 2 diabetes under the brand name Ozempic.
Recognizing its potential in aiding weight loss, researchers developed Wegovy as a dedicated treatment for obesity.

The higher-dose formulation of Wegovy for weight loss surpasses the doses used for diabetes management, harnessing the potential for even greater weight reduction.
In studies, patients treated with Wegovy achieved significant weight loss compared to those receiving a placebo, making it a promising option for individuals struggling with obesity.
Potential for Serious Side Effects: Gastroparesis, Gallbladder Disease, and More
Like Ozempic, Wegovy and its compounded versions, may potentially slow down gastric emptying, leading to gastroparesis.
The medication may also be linked to gallbladder disease.
Patients prescribed Wegovy should be vigilant in monitoring their response to the medication and report any signs of stomach paralysis or other adverse effects to their healthcare provider promptly.

As with any medication, it is crucial for individuals to have open communication with their healthcare team to address any concerns and ensure their well-being throughout the weight loss journey.
What are Compounded Drugs?
Ozempic and Wegovy are both on the FDA drug shortage list.
FDA approved semaglutide medicines
Drug makers have produced compounded versions of Ozempic and Wegovy to combat this shortage.

Compounded versions of drugs are often prescribed when a patient cannot take commercially available medications due to specific allergies, intolerances, or dosage requirements.
In regard to Ozempic and Wegovy, compounding pharmacies have produced alternatives due to the drug shortage.
Compounded drugs, often hailed as a solution during severe drug shortages, come with a slew of inherent dangers that cannot be ignored.
Why are Compounded Drugs Made?
When legitimate pharmaceuticals are scarce due to manufacturing issues, supply chain disruptions, or discontinuation, compounding pharmacies step in to create custom-made medications.
The availability of compounded drugs might seem like a saving grace, but patients should proceed with caution.

Unlike commercially manufactured medications, compounded drugs often lack rigorous testing and quality control measures.
As a result, patients may receive medications of varying potency, purity, and effectiveness, leading to unpredictable health outcomes.
Do You Qualify for the Ozempic Lawsuit?
The Ozempic Lawsuit and Wegovy Lawsuit are potential litigations.
Our attorneys are investigating the potential for litigation on behalf of patients who took these drugs and subsequently developed stomach paralysis and other serious injuries.
If you or a loved one used Ozempic or Wegovy for weight loss and subsequently developed gastroparesis (stomach paralysis) or other related stomach problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw Law for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.

Our Ozempic Lawyers will help you throughout the legal process, completing necessary steps like gathering evidence and assessing damages.
If you are experiencing stomach paralysis symptoms, contact your doctor as soon as possible.
Mitigation is another crucial part in a successful legal claim.
Gathering Evidence for the Ozempic Lawsuit
Evidence is important in product liability lawsuits, especially for a drug as popular and scrutinized as Ozempic.

Evidence in a potential Ozempic Lawsuit claim may include:
- Medical records
- Doctor’s notes
- Prescription records
- Receipts of purchase
- Witness testimony
- Photos and videos
- Personal testimony
- Any other evidence detailing your use of Ozempic and/or Wegovy and subsequent health problems
Your attorney will help you gather relevant evidence, but you can begin this step on your own to best prepare yourself for legal action.
Assessing Damages in the Ozempic Lawsuit
Damages refer to the total amount of losses, economic and non-economic, related to an incident or the use of a dangerous drug.

Potential damages in an Ozempic Lawsuit may include:
- Medical bills
- Future medical expenses
- Lost wages or earning ability
- Pain and suffering
- Emotional damages
- Lost quality of life
- Permanent disability
- Other compensatory and punitive damages
Your Ozempic Lawyer will help you assess and calculate total damages to be included in your legal claim.
Mitigating Injuries for an Ozempic Lawsuit
Mitigation is another crucial part of a successful legal claim.
Mitigating injuries refers to taking action to lessen the impact of Ozempic on your body and life.
Discuss halting use of the drug with a doctor, and explain the symptoms you are experiencing for documentary purposes.

It is also recommended to pursue treatment that your medical professional recommends.
It’s essential that you show proof of attempts to improve your condition.
TruLaw: Investigating the Ozempic Lawsuit
As mentioned, our legal team is investigating the potential safety concerns of Ozempic and Wegovy and strategizing potential legal action against manufacturers for putting consumer health at risk.
If you or a loved one used Ozempic and/or Wegovy, or compounded versions of the drugs, and subsequently developed gastroparesis (stomach paralysis) or other related health problems, you may be eligible to file an Ozempic Lawsuit claim.
Contact TruLaw Law for a free consultation.
You can also use the chatbot on this page for an instant case evaluation.
Ozempic Lawsuit Frequently Asked Questions
-
The Ozempic Lawsuit is a potential litigation.
Ozempic and other similar semaglutide drugs may be linked to gastroparesis (stomach paralysis), gallbladder disease, cyclic vomiting syndrome, and other serious conditions.
The reasons for filing an Ozempic lawsuit may be based on the following key points:
- Consumers were allegedly not sufficiently warned about certain side effects and potential for injury.
- Users of Ozempic, who were later diagnosed with related stomach and gallbladder injuries may be eligible to file a claim.
- Insufficient warning labels on Ozempic and other semaglutide medications (like Wegovy) are claimed to put users at unnecessary risk for certain injuries.
Insufficient Warning About Potential Side Effects
Ozempic lawsuit claims may center around allegations that consumers who took the drug were not sufficiently warned about certain side effects.
These side effects and medical conditions include stomach paralysis, gallbladder disease, cyclic vomiting syndrome, and other stomach problems.
Eligibility to File a Lawsuit
Users of Ozempic, Wegovy, or other semaglutide medications who were later diagnosed with stomach and gallbladder injuries may be eligible to file a claim.
Dangerous drug lawsuits typically aim to pursue compensation for medical expenses, pain and suffering, and other damages.
Insufficient Warning Labels
Many dangerous drug lawsuits claim that the warning labels on medications are insufficient, putting users at unnecessary risk.
Ozempic lawsuits could be potentially filed by people who suffered injuries such as gallbladder disease, stomach paralysis, or cyclic vomiting syndrome.
To conclude, the reasons for filing an Ozempic lawsuit are primarily based on allegations of insufficient warning about potential side effects, the eligibility of patients with related health issues to file a lawsuit, and claims of insufficient warning labels on the medication.
The outcome of these lawsuits could have significant implications for the manufacturer and potentially for the wider pharmaceutical industry.
-
The potential lawsuits against Ozempic and Wegovy for causing Gallbladder Disease and Stomach Paralysis may be based on the following key points:
- Some patients using Ozempic and Wegovy have reported severe gastroparesis (stomach paralysis) gallbladder disease, and cyclic vomiting syndrome.
- Lawsuits claim that consumers were not sufficiently warned about potential side effects, including damage to the gallbladder.
- Users of Ozempic, Wegovy, or other semaglutide medications who were later diagnosed with related gallbladder or bile duct issues may be able to file a lawsuit.
- The Ozempic lawsuits claim that the semaglutide injections can cause acute gallbladder disease.
Reports of Severe Gastroparesis
A recent investigation has found that some patients using Ozempic and Wegovy have suffered from severe gastroparesis, also known as stomach paralysis.
This is a serious condition that can significantly impact a patient’s quality of life and requires medical intervention.
Insufficient Warning About Potential Side Effects
Allegations in Ozempic lawsuits may include claims surrounding whether consumers were not sufficiently warned about the potential side effects of these medications, including damage to the gallbladder.
A lack of sufficient warning could potentially lead to unexpected health issues for patients using these drugs.
Eligibility to File a Lawsuit
Users of Ozempic, Wegovy, or other semaglutide medications who were later diagnosed with related gallbladder disease, gastroparesis, or cyclic vomiting syndrome, or bile duct issues may be eligible to file a claim.
Dangerous drug lawsuits typically aim to pursue compensation for medical expenses, pain and suffering, and other damages.
Claims of Acute Gallbladder Disease
Ozempic semaglutide injections may cause acute gallbladder disease.
This is a serious condition that can lead to severe pain and other complications, and may require surgical intervention.
Consumers may be eligible to file claims against Ozempic and Wegovy related to Gallbladder Disease and Stomach Paralysis.
-
Ozempic has not been approved by the FDA for weight loss.
Wegovy, Ozempic’s “sister drug”, has been approved for weight loss in obese patients.
There has been a significant uptick in off-label use of Ozempic for weight loss in people who are not obese or diagnosed with type 2 diabetes.
Wellness clinics, compounding pharmacies, medical spas, and more have been prescribing Ozempic to people who do not meet criteria for prescriptions, putting them at risk for serious side effects.
Unlawful sales practices regarding Ozempic may also be dwindling the supply of the drug.
-
The active ingredient in Ozempic is semaglutide, a GLP-1 receptor agonist used to treat Type 2 diabetes and, more recently, approved as an anti-obesity medication under the name Wegovy.
-
A compounded drug is a medication often designed to meet specific individual needs or meet demand due to supply chain issues.
Compounded drugs are typically made by mixing different ingredients or altering the dosage form of an existing medication.
-
No, Ozempic is not recalled.
-
Ozempic is typically prescribed by internal medicine specialists, endocrinologists, or healthcare providers specializing in diabetes obesity medicine.
Ozempic has also been reportedly administered at weight loss clinics, wellness clinics, medical spas, and other facilities.
-
Rare side effects of Ozempic include hair loss, renal failure, pancreatitis, intestinal obstruction, gallbladder disease, chronic vomiting syndrome, and gastroparesis, among others.
-
Gastroparesis, commonly known as stomach paralysis, is a condition characterized by delayed emptying of the stomach, leading to digestive issues, nausea, and other symptoms.
-
Gallbladder disease refers to a range of conditions affecting the gallbladder, including gallstones and inflammation (cholecystitis).
It can cause abdominal pain, nausea, and other digestive issues, and in severe cases, may require surgical removal of the gallbladder.
-
Cyclic vomiting syndrome is a rare disorder characterized by recurrent, severe episodes of vomiting with no apparent cause.
These episodes can last for hours or even days, and they are usually followed by symptom-free periods.

Managing Attorney & Owner
With over 25 years of legal experience, Jessica Paluch-Hoerman is an Illinois lawyer, a CPA, and a mother of three. She spent the first decade of her career working as an international tax attorney at Deloitte.
In 2009, Jessie co-founded her own law firm with her husband – which has scaled to over 30 employees since its conception.
In 2016, Jessie founded TruLaw, which allows her to collaborate with attorneys and legal experts across the United States on a daily basis. This hypervaluable network of experts is what enables her to share the most reliable, accurate, and up-to-date legal information with our readers!
Additional Ozempic Lawsuit resources on our website:
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?
At TruLaw, we fiercely combat corporations that endanger individuals’ well-being. If you’ve suffered injuries and believe these well-funded entities should be held accountable, we’re here for you.
With TruLaw, you gain access to successful and seasoned lawyers who maximize your chances of success. Our lawyers invest in you—they do not receive a dime until your lawsuit reaches a successful resolution!
AFFF Lawsuit claims are being filed against manufacturers of aqueous film-forming foam (AFFF), commonly used in firefighting.
Claims allege that companies such as 3M, DuPont, and Tyco Fire Products failed to adequately warn users about the potential dangers of AFFF exposure — including increased risks of various cancers and diseases.
Depo Provera Lawsuit claims are being filed by individuals who allege they developed meningioma (a type of brain tumor) after receiving Depo-Provera birth control injections.
A 2024 study found that women using Depo-Provera for at least 1 year are five times more likely to develop meningioma brain tumors compared to those not using the drug.
Suboxone Tooth Decay Lawsuit claims are being filed against Indivior, the manufacturer of Suboxone, a medication used to treat opioid addiction.
Claims allege that Indivior failed to adequately warn users about the potential dangers of severe tooth decay and dental injuries associated with Suboxone’s sublingual film version.
Social Media Harm Lawsuits are being filed against social media companies for allegedly causing mental health issues in children and teens.
Claims allege that companies like Meta, Google, ByteDance, and Snap designed addictive platforms that led to anxiety, depression, and other mental health issues without adequately warning users or parents.
Transvaginal Mesh Lawsuits are being filed against manufacturers of transvaginal mesh products used to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
Claims allege that companies like Ethicon, C.R. Bard, and Boston Scientific failed to adequately warn about potential dangers — including erosion, pain, and infection.
Bair Hugger Warming Blanket Lawsuits involve claims against 3M — alleging their surgical warming blankets caused severe infections and complications (particularly in hip and knee replacement surgeries).
Plaintiffs claim 3M failed to warn about potential risks — despite knowing about increased risk of deep joint infections since 2011.
Baby Formula NEC Lawsuit claims are being filed against manufacturers of cow’s milk-based baby formula products.
Claims allege that companies like Abbott Laboratories (Similac) and Mead Johnson & Company (Enfamil) failed to warn about the increased risk of necrotizing enterocolitis (NEC) in premature infants.
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?
