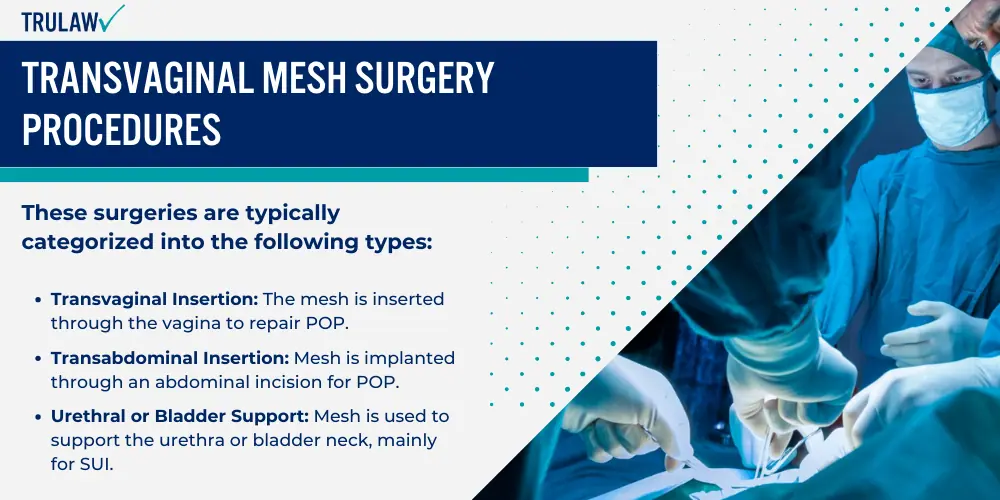
Transvaginal mesh surgery is employed primarily for two distinct medical conditions: Pelvic Organ Prolapse (POP) and Stress Urinary Incontinence (SUI).
These conditions affect the pelvic region and can significantly impact the quality of life.
Pelvic Organ Prolapse
Pelvic Organ Prolapse takes place when pelvic organs like the bladder, rectum, or uterus descend into the vaginal area owing to weakened supporting muscles and tissues.
This condition can lead to discomfort and a variety of symptoms.
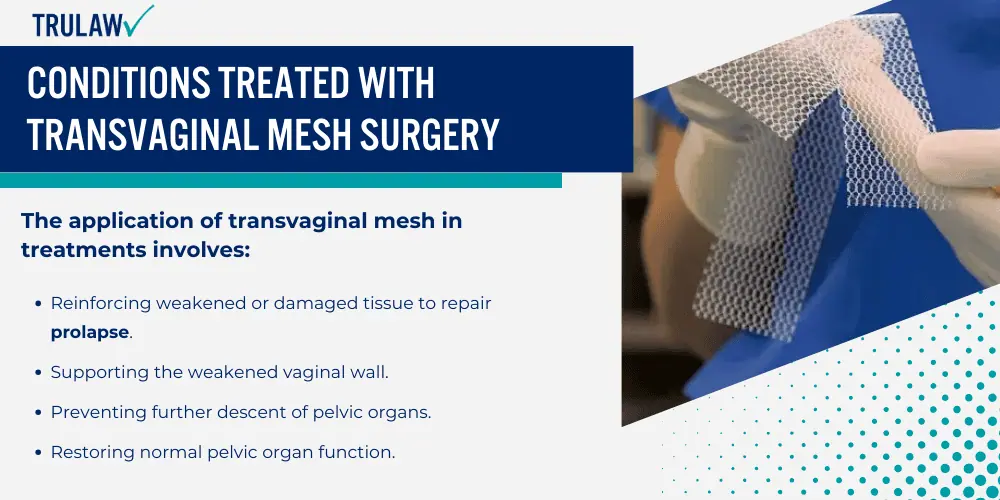
The application of transvaginal mesh in treatments involves:
- Reinforcing weakened or damaged tissue to repair prolapse.
- Supporting the weakened vaginal wall.
- Preventing further descent of pelvic organs.
- Restoring normal pelvic organ function.
Stress Urinary Incontinence
On the other hand, Stress Urinary Incontinence is characterized by the involuntary leakage of urine during physical activities that put pressure on the bladder, such as coughing or exercising.
Transvaginal mesh can provide support to the urethra or bladder neck, which aids in urinary control.
Procedures to rectify stress incontinence may include:
- Placement of a sling to support the urethra.
- Stabilization of the bladder neck.
- Reduction of urinary leakage.
- Improvement of overall urinary function.