Transvaginal mesh is a synthetic polypropylene device surgically implanted through vaginal incision to treat pelvic organ prolapse (when pelvic organs drop from normal position) and stress urinary incontinence (involuntary urine leakage).
These pelvic mesh devices gained FDA approval through 510(k) clearance process in late 1990s and early 2000s based on similarity to previously approved products rather than requiring rigorous clinical trials, allowing rapid market entry without long-term safety data.

Lawsuits exist because internal company documents revealed manufacturers knew about high complication rates including mesh erosion, organ damage, and chronic pain as early as 2005-2009 but continued aggressive marketing to physicians and patients.
Over 100,000 women filed lawsuits between 2010-2022 resulting in more than $8 billion in settlements and jury verdicts, with individual state court cases continuing in 2025 as women discover vaginal mesh complications years after implantation.
What Is Transvaginal Mesh?
Transvaginal mesh is synthetic polypropylene (plastic) material woven into a net-like device surgically implanted through the vagina to provide structural support for weakened vaginal walls and pelvic floor tissues.
It treats two primary medical conditions: pelvic organ prolapse where organs like the bladder, uterus, or rectum drop from normal position causing bulging and discomfort, and stress urinary incontinence where physical activity or pressure causes involuntary urine leakage.
Surgical placement was marketed as “minimally invasive” compared to traditional tissue repair, with procedures typically taking 30-60 minutes and allowing same-day discharge.

Transvaginal mesh exhibits these key characteristics and usage patterns:
- Synthetic polypropylene surgical mesh devices originally developed for hernia repair and adapted for pelvic use without adequate testing for vaginal environment
- Over 300,000 women received transvaginal mesh implants between 2005-2010 according to FDA estimates
- Major manufacturers including Johnson & Johnson/Ethicon, Boston Scientific, C.R. Bard, American Medical Systems (now Endo International), and Coloplast marketed multiple product lines
- Common product names including Gynecare Prolift, Avaulta, Pinnacle, UltraPro, TVT, TVT-O, and Obtryx among many others
- Mesh intended as permanent implant not designed for removal despite manufacturers later claiming removal was always possible
- FDA approved mesh through 510(k) process requiring only “substantial equivalence” to existing devices without independent clinical trial data
Mesh became standard treatment option by mid-2000s with manufacturers providing financial incentives to hospitals and training programs to surgeons, creating rapid adoption without long-term outcome data.
Many women were never informed mesh was synthetic permanent implant or told about alternative treatment options including traditional tissue repair, pelvic floor physical therapy, or pessary devices.
The FDA estimated 300,000 women received these implants between 2005-2010 alone, with complications appearing in 5-19% according to medical literature, though actual complication rates may be higher due to underreporting.
The Rise and Fall of Mesh Implants
Transvaginal mesh became popular in early 2000s as surgeons believed synthetic reinforcement offered better outcomes than traditional tissue repair which had 30-40% recurrence rates.
Manufacturers’ aggressive marketing campaigns emphasized ease of use through surgical mesh “kits” that standardized procedures, shorter surgical time compared to traditional repair, and marketing materials claiming mesh provided superior support preventing prolapse recurrence.
Medical device representatives attended surgeries providing hands-on guidance to surgeons, creating revenue streams and relationships that encouraged adoption.
Key milestones reveal the mesh device timeline:
- 2002-2005: Multiple pelvic mesh products receive FDA 510(k) clearance for pelvic organ prolapse and stress urinary incontinence treatment without clinical trial requirements
- 2005-2008: Peak mesh usage period with widespread adoption and aggressive marketing to OB-GYNs and urogynecologists as superior treatment option
- 2008: FDA issues first safety communication noting rising adverse event reports but takes no regulatory action to restrict use
- 2011: FDA issues second stronger safety communication stating serious complications are “not rare” and orders manufacturers to conduct post-market surveillance studies
- 2013-2016: Major multidistrict litigations consolidate in federal courts as over 100,000 women file lawsuits against manufacturers
- 2016: FDA reclassifies vaginal mesh devices from moderate-risk Class II to high-risk Class III requiring premarket approval demonstrating safety and effectiveness
- 2019: FDA orders vaginal mesh manufacturers to stop selling mesh for pelvic organ prolapse (POP) after determining they could not demonstrate benefits outweighed risks
Problems surfaced as women reported complications within months or years after surgery including vaginal bleeding, chronic pain, mesh erosion through vaginal tissue, painful intercourse, and organ perforation.
The FDA received over 100,000 adverse event reports on transvaginal mesh devices between 2008-2018 documenting serious complications, though actual complication rates were higher because many cases went unreported or doctors failed to connect symptoms with mesh.
The 2019 FDA ban on mesh for pelvic organ prolapse represented vindication for women whose complaints were dismissed for years, confirming that synthetic mesh in vaginal environment created unacceptable risks that manufacturers downplayed or concealed.
Why Manufacturers Face Legal Accountability
Internal company documents obtained through litigation discovery revealed transvaginal mesh manufacturers knew about high complication rates as early as 2005-2009 but continued marketing products as safe and effective.
Specific evidence showed companies received adverse event reports, internal testing revealed mesh degradation and inflammatory responses, and executives discussed complication risks in emails and memos but failed to warn physicians or patients.
Whistleblower testimony and expert witness analysis demonstrated manufacturers prioritized profits over patient safety, choosing to continue selling defective products rather than conducting proper safety testing or issuing warnings.
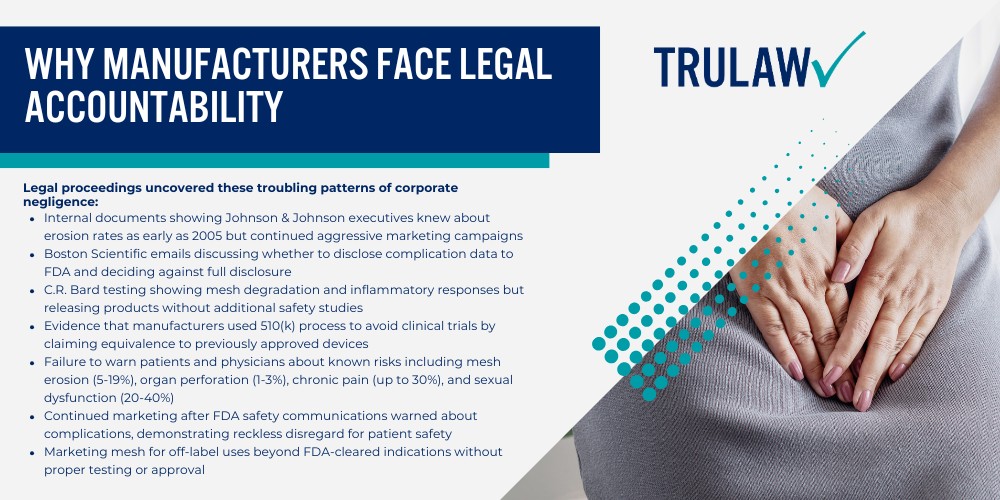
Legal proceedings uncovered these troubling patterns of corporate negligence:
- Internal documents showing Johnson & Johnson executives knew about erosion rates as early as 2005 but continued aggressive marketing campaigns
- Boston Scientific emails discussing whether to disclose complication data to FDA and deciding against full disclosure
- C.R. Bard testing showing mesh degradation and inflammatory responses but releasing products without additional safety studies
- Evidence that manufacturers used 510(k) process to avoid clinical trials by claiming equivalence to previously approved devices
- Failure to warn patients and physicians about known risks including mesh erosion (5-19%), organ perforation (1-3%), chronic pain (up to 30%), and sexual dysfunction (20-40%)
- Continued marketing after FDA safety communications warned about complications, demonstrating reckless disregard for patient safety
- Marketing mesh for off-label uses beyond FDA-cleared indications without proper testing or approval
Over 100,000 lawsuits filed between 2010-2022 resulted in over $8 billion in settlements and jury verdicts, with major manufacturers including Johnson & Johnson/Ethicon paying the largest amounts across consolidated litigation.
Individual settlement amounts ranged from $60,000 to over $400,000 depending on injury severity, with some jury verdicts exceeding $25 million including punitive damages for egregious corporate misconduct.
TruLaw partners with transvaginal mesh litigation leaders who have decades of experience holding manufacturers accountable and recovering compensation for women injured by defective medical devices through both settlement negotiations and trial advocacy when necessary.
Please be advised that any projected or estimated settlement amounts mentioned on this page are general estimations and are not guaranteed.
These figures are based on opinions of legal experts based on the nature of the injuries and estimated costs of damages.
They are meant to provide a general idea of what settlement ranges could look like and should not be taken as definitive expectations for your case.
Contact TruLaw using the chat on this page to receive an instant case evaluation.