The statute of limitations represents a legal deadline that determines whether women affected by transvaginal mesh complications can still pursue compensation for their injuries.
This time limit varies considerably by state and depends on several factors, including when medical complications were discovered rather than when the device was implanted.
Learning these timing requirements serves as the first step in protecting your legal rights.
While many women worry they’ve missed their window to file, numerous exceptions exist that can extend filing deadlines, particularly for those whose vaginal mesh injuries manifested years after surgery.
What Is a Statute of Limitations and Why Does It Matter?
A statute of limitations establishes the maximum time period allowed for filing a lawsuit after an injury occurs or is discovered.
The purpose of this legal time limit is to ensure cases are brought forward while evidence remains fresh, witnesses’ memories are reliable, and relevant documents are still available.
Without these deadlines, defendants would face indefinite exposure to legal claims, making it difficult to defend against allegations from events that occurred many years prior.
In cases following transvaginal mesh surgery, the statute of limitations varies considerably across the United States.
Most states set filing deadlines ranging from one to six years, with the majority of jurisdictions applying a two to three-year period.
However, the starting point for this timeline has become a contentious issue in mesh litigation, as many women don’t immediately realize their symptoms stem from a defective implant.
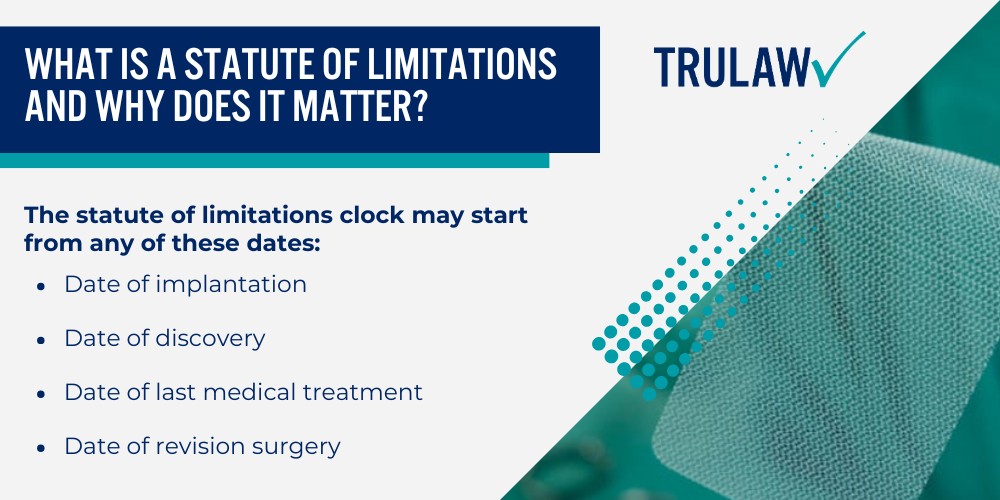
The statute of limitations clock may start from any of these dates:
- Date of implantation: Some states start the clock when the mesh device was surgically placed, regardless of when complications developed
- Date of discovery: Most states now apply the discovery rule, which starts the timeline when the patient knew or reasonably should have known about the injury and its connection to the mesh implant
- Date of last medical treatment: Certain jurisdictions recognize continuing treatment doctrine, which delays the statute during ongoing care for mesh-related issues
- Date of revision surgery: When additional surgeries become necessary to address mesh complications, the statute may reset or extend in some states
The distinction between these starting dates can mean the difference between a viable claim and one that’s forever barred.
A woman who received a defective pelvic mesh implant in 2015 but didn’t discover erosion complications until 2020 could still file a lawsuit in states applying the discovery rule, even though five years have passed since implantation.
According to Cornell Law School’s Legal Information Institute, product liability statutes of limitations balance the interests of injured consumers with the practical needs of manufacturers to have some protection from claims arising from products manufactured many years earlier.
However, medical device cases involving vaginal mesh devices present unique challenges because complications often emerge long after the initial procedure.
Missing the statute of limitations deadline typically results in permanent loss of the right to seek compensation, regardless of how severe the injuries or how strong the evidence of manufacturer wrongdoing.
Courts have limited discretion to hear vaginal mesh cases filed after the deadline expires, making it imperative for affected women to understand and act within the applicable timeframe for their jurisdiction.
If you experienced complications from transvaginal mesh and are concerned about filing deadlines, contact TruLaw using the chat on this page to receive an instant case evaluation.
Determine whether you qualify to file a mesh lawsuit today.
How Transvaginal Mesh Cases Differ from Typical Product Liability Claims
Transvaginal mesh litigation presents unique timing challenges that distinguish it from most product liability cases.
Unlike injuries from defective automobiles or household products that typically manifest immediately or soon after use, mesh complications often emerge years after surgical implantation, creating challenges in determining when the statute of limitations should begin.
The delayed nature of these complications stems from how polypropylene mesh (used for stress urinary incontinence (SUI) and prolapse) degrades within the body.
Research published in October 2024 revealed that polypropylene begins to degrade within just 60 days of implantation, with further structural breakdown evident by 180 days.
However, the symptoms associated with this degradation may not become apparent or severe enough for diagnosis for months or even years afterward.

Women often struggle to connect their symptoms to mesh implants for several reasons:
- Gradual symptom onset: Pain, erosion, and other complications often develop slowly over time rather than appearing suddenly after surgery
- Medical misdiagnosis: Doctors may initially attribute symptoms to other conditions such as infection, hormonal changes, or unrelated pelvic floor disorders
- Lack of manufacturer warnings: Companies like Johnson & Johnson’s Ethicon unit, Boston Scientific, and Coloplast faced allegations they failed to adequately warn patients, leaving both patients and physicians unaware of potential mesh-related complications
- Multiple consultations required: Women frequently see several healthcare providers and undergo various tests before they learn that vaginal mesh complications occurred
The FDA’s 2011 Safety Communication acknowledged that serious complications associated with transvaginal mesh placement to treat pelvic organ prolapse were “not rare,” contrary to earlier statements.
This update came after reviewing adverse event reports and scientific literature, but many women had already experienced complications without recognizing their connection to the mesh device.
Additionally, transvaginal mesh often requires revision surgeries to address complications or remove deteriorated mesh material.
In many jurisdictions, these subsequent surgical procedures can reset or extend the statute of limitations.
A woman who underwent mesh implantation in 2016 but required erosion repair surgery in 2022 may have a new two or three-year window starting from the revision surgery date, depending on her state’s laws.
The inflammatory response triggered by degrading polypropylene can continue causing new injuries years after initial placement.
This ongoing harm distinguishes mesh cases from discrete injury events, as women may suffer from progressive complications including chronic pelvic pain, recurrent infections, organ perforation, nerve damage, and sexual dysfunction that worsen over time rather than remaining static.














