Our Transvaginal Mesh attorney at TruLaw is dedicated to supporting clients through the process of filing a Transvaginal Mesh lawsuit.
With extensive experience in product liability cases, Jessica Paluch-Hoerman and our partner law firms work with litigation leaders and medical experts to prove how defective mesh implants caused you harm.
TruLaw focuses on securing compensation for medical expenses, revision surgeries, pain and suffering, lost income, and other damages resulting from your transvaginal mesh injuries.
We understand the physical and emotional toll that Transvaginal Mesh complications have on your life and provide the personalized guidance you need when seeking justice.
Meet the Lead Transvaginal Mesh Attorney at TruLaw
Meet our lead Transvaginal Mesh attorney:
- Jessica Paluch-Hoerman: As founder and managing attorney of TruLaw, Jessica brings her experience in product liability and personal injury to her client-centered approach by prioritizing open communication and personalized attention with her clients. Through TruLaw and partner law firms, Jessica has helped collect over $3 billion on behalf of injured individuals across all 50 states through verdicts and negotiated settlements.
How much does hiring a Transvaginal Mesh lawyer from TruLaw cost?
At TruLaw, we believe financial concerns should never stand in the way of justice.
That’s why we operate on a contingency fee basis—with this approach, you only pay legal fees after you’ve been awarded compensation for your injuries.
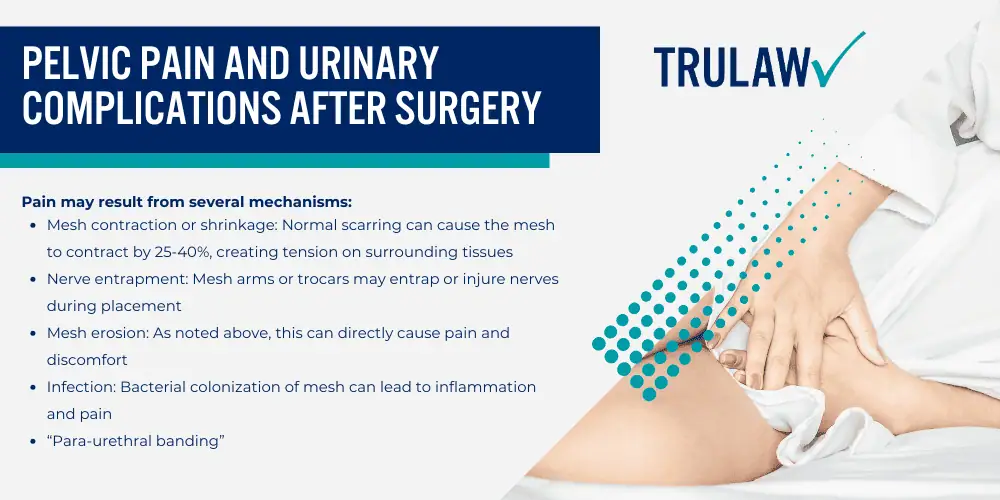
If you or a loved one experienced pain, bleeding, infection, organ perforation, mesh erosion, or other complications from transvaginal mesh implants, you may be eligible to seek compensation.
Contact TruLaw using the chat on this page to receive an instant case evaluation and determine whether you qualify to join others in filing a Transvaginal Mesh lawsuit today.