This synthetic material helps to support weakened or damaged tissue surrounding the pelvic organs; however, various complications can arise from its use.

Several manufacturers produce this mesh with differing brands available on the market.
Transvaginal mesh alternatives exist outside of opting for implants, such as native tissue repair or biologic meshes derived from animal tissues.
What is Transvaginal Mesh?
Transvaginal mesh is a medical device commonly used in surgeries to treat conditions like stress urinary incontinence (SUI) and pelvic organ prolapse (POP).
It involves making an incision in the vaginal wall, through which the mesh is inserted, offering much-needed support for weakened or damaged tissue.
Made of absorbable or permanent material, this surgical mesh provides reinforcement to the vaginal wall and bladder.
Despite its prevalent usage, there have been higher instances of problems related to transvaginal mesh compared to other treatments.
Therefore, it’s imperative for women considering this procedure to fully understand its potential benefits and risks.
Uses of Transvaginal Mesh
When the pelvic floor weakens, it can no longer adequately support pelvic organs like the bladder or uterus.
This lack of support causes these organs to drop, a condition known as POP.
Transvaginal mesh provides much-needed reinforcement to the weakened vaginal walls.
Another common use of this product is for SUI treatment.
This condition leads to unintentional urine leakage when pressure increases on the bladder due to actions like laughing, sneezing or exercising.
Surgeons implant transvaginal mesh as a sling around the urethra or bladder neck, thereby controlling involuntary urine flow.
Unlike traditional surgery methods that use stitches alone, surgeons turn to synthetic polypropylene meshes because they offer increased durability and strength after being surgically implanted through the vagina.
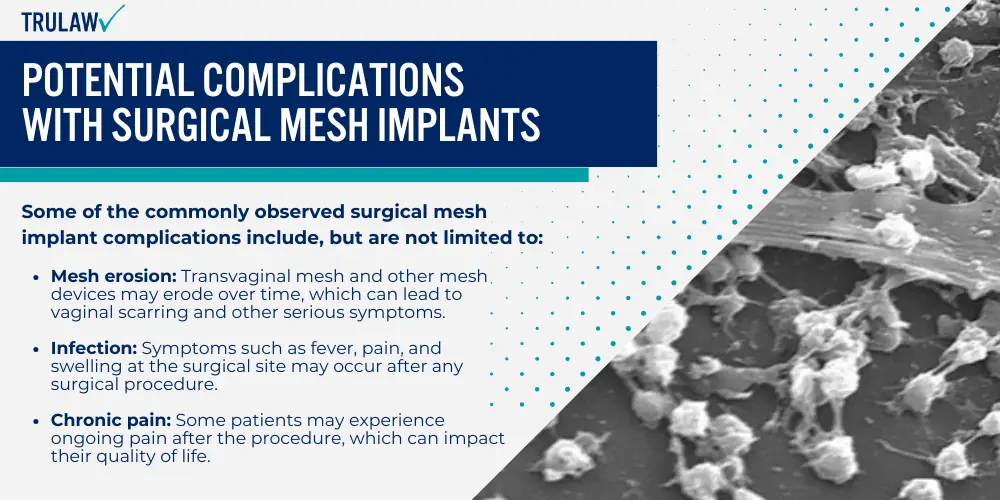
Complications with Transvaginal Mesh Implants
Unfortunately, these implants come with a range of complications that can cause significant discomfort and potential health risks.
The most common problem faced by patients is pain, which could be due to the implant itself or as a result of surgical procedures.
One serious concern associated with transvaginal mesh implants is mesh erosion—an issue flagged by the FDA due to its frequency among patients.
Mesh erosion occurs when the synthetic materials used in these devices wear away at surrounding tissues.
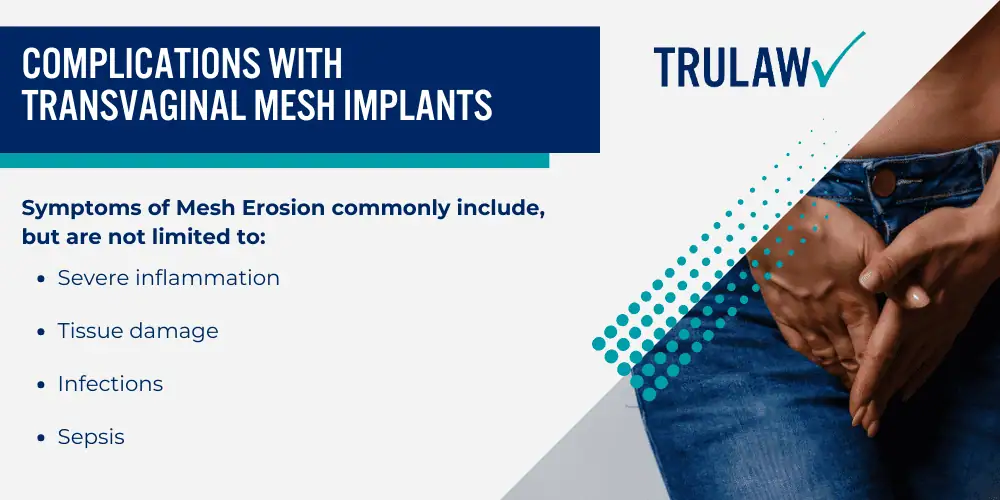
Symptoms of Mesh Erosion commonly include, but are not limited to:
- Severe inflammation
- Tissue damage
- Infections
- Sepsis
This condition often requires additional surgery for correction or removal of the eroded material.
Additionally, during the placement procedure for transvaginal mesh devices, there’s an inherent risk of injury to adjacent organs like the bladder or bowel which might require immediate medical intervention.
Ultimately, monitoring these potential difficulties plays a crucial role in ensuring patient safety post-implantation process while managing pelvic floor disorders effectively.
Manufacturers and Brands of Transvaginal Mesh
Several companies manufacture transvaginal meshes, including global medical device providers.
These manufacturers design and produce a variety of mesh brands to suit different patient needs and complications.
One such brand is Boston Scientific’s Advantage Sling System, utilized widely for its effectiveness in treating pelvic organ prolapse and stress urinary incontinence.
However, after the FDA directive in 2019 barring the sale of these devices for transvaginal repair of pelvic organ prolapse within the United States, many products are no longer available domestically.
Despite these changes in availability, numerous other brands of this vital device exist worldwide with similar designs and functionalities.
Alternatives to Transvaginal Mesh
Exploring alternatives to transvaginal mesh is crucial for women seeking treatment for conditions like pelvic organ prolapse and stress urinary incontinence.
Here are some other alternatives to transvaginal mesh that medical professionals may suggest:
- Native Tissue Repair: This surgical procedure uses a woman’s own tissues to support pelvic organs experiencing prolapse.
- Prolapse Repairs without Mesh: Surgeons make repairs using stitches alone, without the addition of synthetic materials or biologic mesh.
- Pessaries: These are non-surgical devices inserted into the vagina to support prolapsed organs.
- Pelvic Floor Therapy: Specialist therapists provide exercises designed to strengthen the pelvic floor muscles.
- Lifestyle modifications: Doctors may recommend changes such as weight loss, bowel management strategies, and quitting smoking.
- Non-Surgical Management with Vaginal Estrogen: This treatment option involves taking estrogen to improve the health of vaginal tissues and potentially slow prolapse progression.
- Laparoscopic Sacrocolpopexy: A minimally invasive surgical technique used for repairing pelvic organ prolapse, usually done with a synthetic polypropylene mesh attached from the cervix or top of the vagina up towards the sacrum (a bone at the base of your spine).
- Mid-Urethral Sling Procedure: It’s primarily used for treating stress urinary incontinence by placing a mesh sling under the urethra to support it and help keep it closed during physical activity.
- Colpocleisis: A procedure done for older adults who aren’t sexually active anymore; it involves closing off or removing parts of their vagina.
Lawsuits Involving Transvaginal Mesh
Transvaginal mesh lawsuits have risen significantly over the years due to numerous complications experienced by patients.
These complications often include severe discomfort, bleeding, infection, and even organ perforation.
The legal claims against companies manufacturing transvaginal mesh accuse them of negligence along with breaches in warranty, and defects in design and manufacturing processes.
One notable settlement among these lawsuits stands as one of the largest to date.
The initiation of most transvaginal mesh lawsuits arose following a 2011 communication from the Food and Drug Administration concerning the use of surgical mesh for pelvic organ prolapse repair.
Consequently, these settlements contributed to an approximate $8 billion payout in personal injury cases related to transvaginal mesh complications.