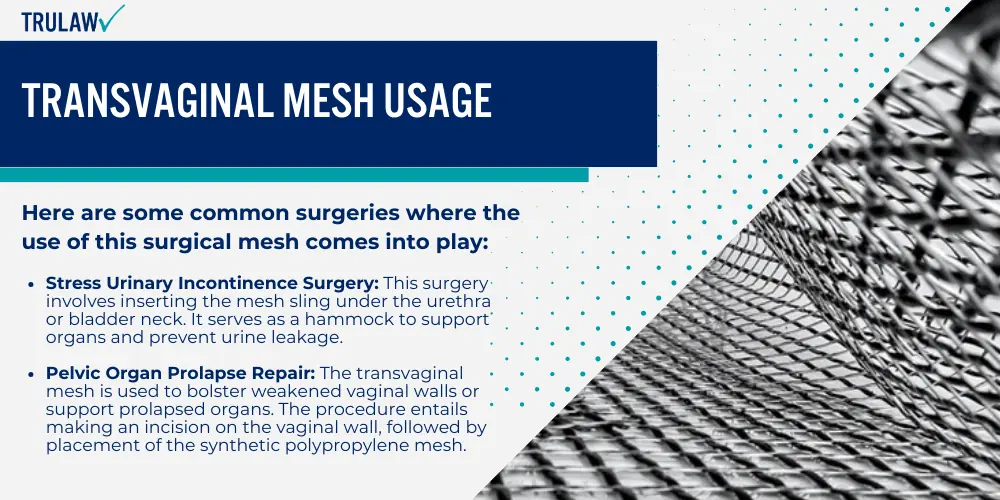
Transvaginal mesh is a medical device designed to provide internal tissue support when it’s weakened or damaged.

It’s similar to a net in structure and can be made from synthetic materials like polymers or biological material such as animal tissue.
The mesh actually acts as a scaffold that allows new tissues to grow through its pores, thus providing long-term reinforcement for the affected area.
Types of Transvaginal Mesh Products
- Polypropylene mesh: is a synthetic type of transvaginal mesh typically used for long-term support, particularly in patients with severe pelvic organ prolapse or stress urinary incontinence.
- Biological mesh: is derived from animal tissue and is usually considered for patients who may have adverse reactions to synthetic materials.
- Composite mesh: combines different types of materials, designed to meet specific individual patient needs and offer customized care.
- Absorbable and non-absorbable mesh: the difference between these types of transvaginal mesh products depends whether temporary or long-lasting support is necessary.
Remember, the type of mesh selected for a procedure relies heavily on a patient’s unique medical condition, the severity of their symptoms, their overall health, and their surgeon’s expertise and preference.
Therefore, it’s vital for patients to discuss the options with their healthcare providers thoroughly before making a decision.
Manufacturers of Pelvic Mesh Products
Several major manufacturers have produced transvaginal mesh products designed to assist in the surgical treatment of pelvic organ prolapse and stress urinary incontinence.
These products can differ greatly in their structure, design, and materials used, which can affect their efficacy and potential complications.
Let’s take a look at the major manufacturers in the industry and their respective pelvic mesh products:
- Gynecare Prolift: A pelvic mesh product designed by Ethicon, (a subsidiary of Johnson & Johnson), to provide support and repair pelvic organ prolapse.
- Advantage Transvaginal Mid-Urethral Sling System: A sling system created by Boston Scientific to address stress urinary incontinence in women.
- Apogee Vaginal Vault Prolapse Repair System: A system designed by American Medical Studies to correct vaginal vault prolapse.
- Avaulta Plus: A biologic implant designed by C.R. Bard for the treatment of pelvic organ prolapse.
In April 2019, the FDA ordered all manufacturers to cease the production and distribution of surgical mesh intended for transvaginal repair of pelvic organ prolapse.
Despite this, surgical mesh products may still be used for other procedures, such as treatment of stress urinary incontinence and abdominal repair of pelvic organ prolapse.
It is crucial to discuss all potential treatment options, along with their associated risks and benefits, with your healthcare provider.