New Suboxone Lawsuit: Teeth Decay Linked to Opioid Treatment
- Last Updated: June 12th, 2025

Attorney Jessica Paluch-Hoerman, founder of TruLaw, has over 28 years of experience as a personal injury and mass tort attorney, and previously worked as an international tax attorney at Deloitte. Jessie collaborates with attorneys nationwide — enabling her to share reliable, up-to-date legal information with our readers.
Legally Reviewed
This article has been written and reviewed for legal accuracy and clarity by the team of writers and legal experts at TruLaw and is as accurate as possible. This content should not be taken as legal advice from an attorney. If you would like to learn more about our owner and experienced injury lawyer, Jessie Paluch, you can do so here.
Fact-Checked
TruLaw does everything possible to make sure the information in this article is up to date and accurate. If you need specific legal advice about your case, contact us by using the chat on the bottom of this page. This article should not be taken as advice from an attorney.
New Suboxone Lawsuit: Teeth Decay Linked to Opioid Treatment
On this page, we’ll discuss an overview of the new Suboxone lawsuit, tooth decay side effects linked to Suboxone, who qualifies to file a Suboxone lawsuit, and much more.

Intro to the Suboxone Lawsuit
Suboxone is a prescription drug manufactured by Indivior used to treat opioid addiction.
The drug contains two active ingredients:
- Buprenorphine: A partial opioid agonist that reduces cravings for opioids and prevents withdrawal symptoms. However, it can also cause addiction and dependence.
- Naloxone: An opioid antagonist that blocks the effects of opioids and reverses an overdose.
While these drugs have been used for decades, they have recently been linked to serious health problems, including addiction, dependency, and death.
If you or a loved one has experienced side effects from Suboxone, contact TorHoerman Law today using the chat on this page for a free consultation.
Table of Contents
Developments in the Suboxone Lawsuit
The Suboxone lawsuit has seen several crucial developments, particularly around the issue of tooth decay claims.

Plaintiffs argue that if they had been aware of these risks, they could have taken preventive measures or chosen alternative treatments
Here’s a summary of the latest suboxone lawsuit updates:
- January 2024: The U.S. Judicial Panel on Multidistrict Litigation is scheduled to consider consolidating 15 lawsuits related to Suboxone tooth decay claims into multidistrict litigation (MDL).
- December 2023: The FDA announced the final approval and implementation of required labeling updates to address the evolving opioid crisis. This follows reports and a study confirming that the sublingual buprenorphine/naloxone in Suboxone, due to its acidic nature, increases the risk of dental problems like cavities and tooth loss.
- November 27, 2023: Legal representatives petitioned to consolidate all federal Suboxone lawsuits into multidistrict litigation. As of this date, 14 new Suboxone tooth decay lawsuits were filed against Indivior in federal courts.
- October 23, 2023: Indivior agreed to pay $385 million to settle Suboxone monopoly lawsuits filed by drug manufacturers. This settlement is separate from the ongoing tooth decay claims.
- Earlier Developments: Indivior had previously settled a lawsuit for $102.5 million related to antitrust allegations. Additionally, they reached a $30 million settlement in August 2023 with healthcare plans that had brought a federal antitrust lawsuit against the company.
The core of these lawsuits is the claim that Indivior failed to adequately warn users of the risks of severe tooth decay and other dental problems associated with Suboxone, particularly before adding a warning in 2022.
In the last two weeks of November 2023 alone, 14 new Suboxone product liability lawsuits were filed in federal courts.
Of these, eight were filed in the Northern District of Ohio, which currently holds the most pending Suboxone cases of any district.
1. January 25, 2024: U.S. Judicial Panel on Multidistrict Litigations
The U.S. Judicial Panel on Multidistrict Litigation is set to consider consolidating 15 lawsuits related to Suboxone tooth decay claims.
These lawsuits were filed in five different jurisdictions.
The lawsuits allege that Indivior, the manufacturer of Suboxone, failed to adequately warn users about the potential dental side effects, such as tooth decay, tooth loss, infections, cracked teeth, and cavities.
2. October 23, 2023: Indivior agreed to pay $385 million
This settlement reached by U.S. lawmakers claimed that Indivior illegally suppressed generic competition.
In October 2023, Indivior agreed to pay $385 million to settle Suboxone lawsuits filed by drug wholesalers.
This settlement was reached with U.S. lawmakers and resolved claims that Indivior illegally suppressed generic competition for Suboxone.
3. Antitrust Allegations: Indivior To Pay $102.5 million
In a widespread settlement involving 41 states and the District of Columbia, Indivior has agreed to pay $102.5 million.
This settlement addresses issues related to Indivior’s business practices and the impact on Suboxone’s availability and pricing in these states.
Regarding the settlement involving 41 states and the District of Columbia, where Indivior agreed to pay $102.5 million, the available information from the sources does not specifically confirm this detail.
However, it is noted that Indivior and Reckitt Benckiser faced various legal challenges, including an antitrust class-action lawsuit and settlements with the Federal Trade Commission totaling $60 million, related to deceptive marketing practices and limiting generic competition for Suboxone.
Why are Suboxone Lawsuits Being Filed?
The lawsuits primarily revolve around the allegations that Indivior, the manufacturer of Suboxone, failed to warn about the risks of dental injuries.

This move comes in the wake of numerous reports of severe tooth decay experienced by Suboxone users.
Key points to consider about the suboxone lawsuit include:
- Specific dental issues associated with Suboxone;
- Nature of the warning provided by the manufacturer;
- Outcome of previous related lawsuits; and
- Potential impact on patients’ health and financial situations.
The Suboxone lawsuit is a significant legal matter due to its assertions about the drug’s adverse effects and the manufacturer’s alleged failure to inform.
This allegation is critical as it can potentially affect current and future patients, healthcare professionals, and the pharmaceutical industry.
What is Suboxone?
Suboxone is a medication used to aid individuals struggling with opioid addiction.
However, many people have reported severe dental issues, including tooth decay, erosion, dry mouth, gum problems, tooth fractures, and infections, as a result of taking Suboxone.
The lawsuits claim that the manufacturer, Indivior, did not adequately warn people about the risk of dental problems associated with the medication.
Some studies have suggested a link between Suboxone use and dental issues, and the FDA has issued warnings about the dental risks of the medication.
The statute of limitations for filing a suboxone lawsuit related to health conditions such as tooth decay varies from state to state.
Those who have suffered psychologically, emotionally, or financially due to these issues, contact TorHoerman Law using the chat on this page for an instant case evaluation.
The Intricacies of Suboxone Litigation
Suboxone litigation involves navigating complex medical and legal landscapes to advocate for clients’ rights.

Health Conditions Linked to Suboxone
Understanding the health conditions linked to Suboxone is crucial for building a strong legal case.
Health conditions potentially associated with Suboxone include:
- Respiratory issues and potential risk of overdose due to the drug’s effects on breathing.
- Liver damage, including hepatitis and liver failure, may be linked to the medication’s active ingredients.
- Dependency and withdrawal symptoms can occur even with prescribed use.
Patients who have been prescribed Suboxone as part of their treatment plan may not have been fully informed of the potential risks associated with its use.
TruLaw is actively investigating claims that the manufacturer of Suboxone failed to provide adequate warnings about the dangers of the drug, which could have led to preventable injuries or even fatalities.
Qualifications for Filing a Suboxone Lawsuit
Determining who qualifies to file a Suboxone lawsuit is a critical step in seeking justice.
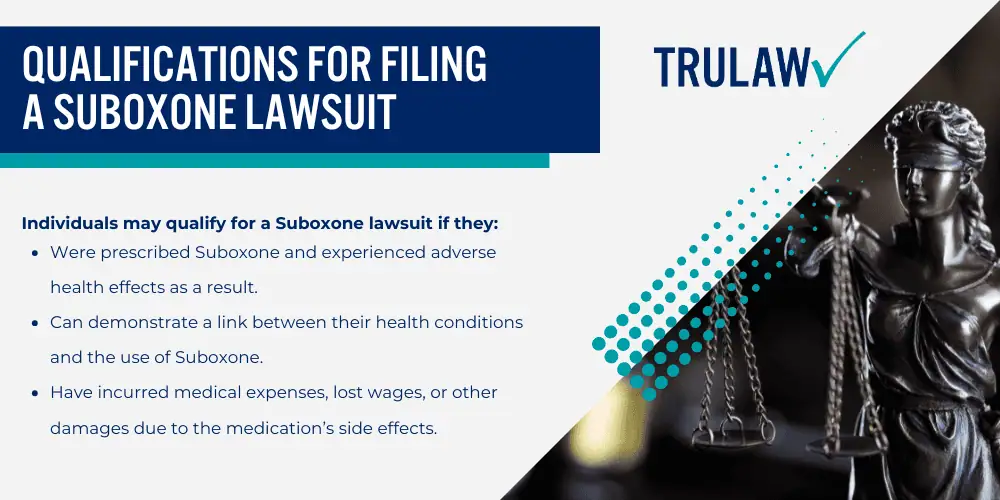
Individuals may qualify for a Suboxone lawsuit if they:
- Were prescribed Suboxone and experienced adverse health effects as a result.
- Can demonstrate a link between their health conditions and the use of Suboxone.
- Have incurred medical expenses, lost wages, or other damages due to the medication’s side effects.
TruLaw’s legal team is skilled at assessing the merits of a potential Suboxone lawsuit, taking into account the specifics of each individual’s circumstances.
The firm’s attorneys work closely with medical experts to establish causation between Suboxone use and the client’s health issues, which is a crucial element in building a successful legal claim.
Average Settlement Amounts in Suboxone Litigation
Average settlement amounts in Suboxone litigation can vary based on the details of each case.

Factors influencing settlement amounts include:
- The severity of the health condition and its impact on the individual’s quality of life.
- The extent of medical treatment required and associated costs.
- The strength of the legal claim and the ability to prove causation.
While settlements in Suboxone litigation can provide financial relief to those affected, they also serve a broader purpose.
They can act as a deterrent to pharmaceutical companies, encouraging them to prioritize patient safety and transparency.
TruLaw is committed to securing fair compensation for its clients and holding negligent parties accountable for their actions.
Past Legal Proceedings in Suboxone Litigation
Staying informed about both upcoming and past legal proceedings is essential for those involved in Suboxone litigation.

RB Group’s $700 Million Settlement: Suboxone Controversy
These settlements have significant implications for those prescribing Suboxone, especially concerning the availability of generic Suboxone tablets and sublingual strips.
Details of previous Suboxone litigations include:
- Civil Agreement Resolution: RB Group’s $700 million settlement addressing claims of misleading marketing affecting government health programs.
- Federal and State Allocation: Distribution of settlement funds – $500 million to the federal government and up to $200 million to participating states.
- Allegations Without Liability Admission: Highlighting that these are allegations with no established liability.
From 2010 to 2014, the United States alleges that RB Group, through its branches, engaged in questionable practices with Suboxone.
The allegations against RB Group include:
- Promotion to Certain Physicians: RB Group is accused of promoting Suboxone, a branded drug, to doctors who prescribed it without the necessary support. These prescriptions were often unsafe or unnecessary, raising concerns about their use.
- Misleading Claims About Suboxone Film: The company allegedly pushed the film version of Suboxone with false claims. They stated it was less prone to abuse and safer for children than other forms. This is critical, considering issues like deteriorating dental health and serious dental problems linked to Suboxone.
- Efforts to Delay Generic Competition: In a move scrutinized by a federal grand jury, RB Group claimed in 2012 that they discontinued Suboxone tablets due to safety, an attempt to stall generic versions and control pricing.
The questionable practices leading up to the ongoing suboxone litigations focused on false marketing claims and the drug’s impact, like severe tooth decay and dental erosion.
Suboxone lawyers at TruLaw are closely monitoring this case, as it involves serious allegations of false marketing and its impact on those using Suboxone for reducing withdrawal symptoms.
The federal court’s involvement underscores the gravity of these allegations and their potential impact on public health and healthcare costs.
Scientific Research on Suboxone and Dental Injuries
These investigations reveal that patients using Suboxone experience a higher frequency of dental issues like cavities, tooth decay, and gum disease compared to those using other opioid dependency treatments.

The acidic nature of Suboxone, particularly when dissolved in the mouth, is believed to weaken tooth enamel and detrimentally impact oral health over time.
Key studies and publications on Suboxone dental injuries include:
- Brown University Child & Adolescent Psychopharmacology Update (2022): This update from Brown University warned about dental problems associated with buprenorphine medicines dissolved in the mouth, a category including Suboxone.
- Drug and Therapeutics Bulletin (2023): This bulletin addressed the dental adverse effects of sublingual buprenorphine and naloxone, emphasizing the risks of long-term use.
- University of British Columbia (2022): Researchers at the University of British Columbia studied the association between sublingual buprenorphine-naloxone exposure and dental disease, providing empirical evidence of the risks.
- Clinical Pharmacology & Therapeutics (2011): This earlier study focused on the induction of opioid-dependent individuals onto buprenorphine and buprenorphine/naloxone soluble films, offering insights into the onset of dental issues in patients.
- Expert Opinion on Drug Safety (2023): A pharmacovigilance study in this publication explored the connection between sublingual/buccal buprenorphine and dental problems, highlighting the safety concerns for patients.
- Reactions Weekly (2013): This report included a case study of dental caries associated with buprenorphine/naloxone, illustrating the real-world impact of these side effects.
- Journal of Gerontological Nursing (2022): This journal article explored opioid use disorder and older adults, discussing treatment options and their implications, including Suboxone’s dental risks.
The studies and reports collectively underscore the need for increased awareness and precautionary measures for patients using Suboxone, especially those with pre-existing dental issues or at higher risk of dental complications.
Buprenorphine: Drug Facts
Buprenorphine-containing medicines are approved to treat opioid use disorder (OUD), and one product is approved to treat pain.

These medicines are available as single-ingredient products and in combination with naloxone.
Buprenorphine medicines are marketed under the brand names:
- Belbuca;
- Bunavail;
- Cassipa;
- Suboxone;
- Subutex; and
- Zubsolv.
They are also available as generics.
Buprenorphine is an opioid and works by changing the way the brain and nervous system respond to pain.
In patients taking buprenorphine for OUD, it reduces opioid withdrawal symptoms and the desire to use opioids without causing the cycle of highs and lows associated with opioid misuse or abuse.
At proper doses, buprenorphine also decreases the pleasurable effects of other opioids, making continued opioid use less appealing.
When combined with counseling and other behavioral therapies, this comprehensive medication-assisted treatment (MAT) approach is often the most effective way of treating OUD.
It can help sustain recovery and prevent or reduce opioid overdose
Common side effects of buprenorphine include:
- Headache;
- Nausea;
- Vomiting;
- Constipation;
- Pain;
- Increased sweating;
- Insomnia; and
- Other severe health conditions
Buprenorphine Medicines and Dental Health Concerns
The use of buprenorphine drugs, particularly those dissolved orally like Suboxone sublingual films and strips, has surged in recent years.

From 2014 to 2020, the number of these prescriptions dispensed in the United States climbed from 11 million to 16 million.
Dental Issues Associated with Buprenorphine Medicines
A concerning number of dental problems have been linked to these medications:
- Reported Cases: The FDA has recorded 305 cases of dental problems, with 131 classified as serious, related to buprenorphine medicines.
- Underreporting Possibility: This figure only includes reported cases, suggesting the actual number might be higher.
- Age Range of Affected Patients: Patients as young as 18 and averaging 42 years of age have suffered dental issues.
- Reasons for Use: While most cases involved patients using the medicine for Opioid Use Disorder (OUD), 28 cases were reported in those using it for pain treatment.
- No Prior Dental History: In 26 instances, patients had no previous history of dental problems.
Timeframe and Severity of Dental Problems
The onset and impact of these dental issues vary:
- Early Onset: Some patients reported problems as early as 2 weeks after starting treatment.
- Median Time to Diagnosis: On average, dental problems were diagnosed about 2 years after the commencement of treatment.
- Healthcare Professional Reports: Many cases were documented by medical providers, noting extensive dental adverse events.
Treatment of Dental Issues
The nature of dental treatments highlights the severity of these issues:
- Common Treatment: Tooth extraction or removal, reported in 71 cases.
- Other Dental Procedures: Patients require root canals, dental surgery, and restorative procedures like crowns and implants.
- Multiple Teeth Affected: In 113 of the 305 cases, more than one tooth was impacted.
Legal and Regulatory Implications
These findings raise significant legal and regulatory concerns:
- Suboxone Dental Decay Lawsuit: Patients are seeking legal recourse through Suboxone teeth lawsuits and class action lawsuits.
- Federal Government and Court Involvement: The United States District Court and the federal government are key players in addressing these issues, potentially leading to a Suboxone settlement.
- False Claims Act Lawsuit and Illegal Kickback Scheme Allegations: These cases add to the complexity of the situation, questioning the marketing and prescribing practices of Suboxone and generic versions.
Impact of the Suboxone Lawsuit
The Suboxone lawsuit marks a critical development in addressing opioid crisis complexities, particularly focusing on the consequences of Suboxone used in opioid dependence treatment.

Suboxone Tooth Decay Lawsuit Victims
Victims affected by Suboxone may find the following aspects of the lawsuit particularly relevant:
- Eligibility for Compensation: Those who have experienced severe dental injuries, including tooth decay, due to prescribed Suboxone film or tablets.
- Addressing Dental Health Issues: Recognition of the need for treatments for oral infections, tooth extractions, and other dental injuries caused by Suboxone.
- Legal Support: Access to Suboxone tooth decay lawyers specializing in representing such cases.
Legal Implications for Indivior and Reckitt Benckiser
This lawsuit brings to light significant issues for the implicated companies:
- Failure to Warn: Allegations against these companies for not informing users and medical providers about Suboxone’s potential dental side effects.
- Financial and Legal Repercussions: Potential consequences if found guilty, impacting their operations and legal standing.
Changes in Pharmaceutical Industry Practices
The lawsuit could lead to broader implications in the industry:
- Enhanced Accountability: A push for more transparent marketing and patient safety measures.
- Regulatory Changes: Possible reforms in how pharmaceutical companies report and manage side effects of drugs like buprenorphine medications.
Commonly Reported Injuries: Suboxone Lawsuit
The range of reported dental injuries highlights significant concerns:
- Tooth Loss and Decay: Direct impacts on dental health due to Suboxone use.
- Oral Infections and Cavities: Additional complications arising from the drug’s side effects.
- Other Dental Issues: A spectrum of dental problems linked to Suboxone usage needs attention in the lawsuit.
The Broader Impact
This lawsuit extends beyond individual claims, affecting the entire pharmaceutical sector:
- Increased Regulatory Scrutiny: Potential for more stringent oversight on pharmaceutical companies.
- Raising Awareness: Highlighting issues like accidental pediatric exposure and the need for safer prescribing practices.
- Legal Precedents: Setting examples for future cases involving pharmaceutical companies and user safety.
In summary, the Suboxone lawsuit is a significant milestone, not just for the victims seeking justice but also for the broader implications it holds for pharmaceutical practices, legal standards, and patient safety in the context of opioid addiction treatment.
The #1 Suboxone Lawyer: TruLaw
TruLaw offers unparalleled expertise and advocacy in Suboxone litigation services.

Our team of experienced attorneys is well-versed in the complexities of pharmaceutical litigation, providing our clients with the support they need to navigate their legal challenges.
TruLaw provides comprehensive Suboxone litigation services:
- Expert legal counsel and representation for individuals affected by Suboxone.
- Strategic advice and support throughout the litigation process, from initial consultation to resolution.
- A commitment to securing justice and fair compensation for those impacted by Suboxone side effects.
Partnering with TruLaw ensures that clients receive informed and strategic legal counsel in the area of Suboxone litigation.
Our dedication to staying at the forefront of legal development enables us to offer advice that is not only current but also forward-thinking.
Whether you are facing a Suboxone dispute or seeking to understand your legal options, TruLaw is your trusted partner in pharmaceutical litigation.
Staying informed and prepared is essential for success.
With the support and guidance of TruLaw, clients can confidently face the challenges of Suboxone litigation and secure their legal rights.

Managing Attorney & Owner
With over 25 years of legal experience, Jessica Paluch-Hoerman is an Illinois lawyer, a CPA, and a mother of three. She spent the first decade of her career working as an international tax attorney at Deloitte.
In 2009, Jessie co-founded her own law firm with her husband – which has scaled to over 30 employees since its conception.
In 2016, Jessie founded TruLaw, which allows her to collaborate with attorneys and legal experts across the United States on a daily basis. This hypervaluable network of experts is what enables her to share the most reliable, accurate, and up-to-date legal information with our readers!
Additional Suboxone Lawsuit resources on our website:
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?
At TruLaw, we fiercely combat corporations that endanger individuals’ well-being. If you’ve suffered injuries and believe these well-funded entities should be held accountable, we’re here for you.
With TruLaw, you gain access to successful and seasoned lawyers who maximize your chances of success. Our lawyers invest in you—they do not receive a dime until your lawsuit reaches a successful resolution!
AFFF Lawsuit claims are being filed against manufacturers of aqueous film-forming foam (AFFF), commonly used in firefighting.
Claims allege that companies such as 3M, DuPont, and Tyco Fire Products failed to adequately warn users about the potential dangers of AFFF exposure — including increased risks of various cancers and diseases.
Depo Provera Lawsuit claims are being filed by individuals who allege they developed meningioma (a type of brain tumor) after receiving Depo-Provera birth control injections.
A 2024 study found that women using Depo-Provera for at least 1 year are five times more likely to develop meningioma brain tumors compared to those not using the drug.
Suboxone Tooth Decay Lawsuit claims are being filed against Indivior, the manufacturer of Suboxone, a medication used to treat opioid addiction.
Claims allege that Indivior failed to adequately warn users about the potential dangers of severe tooth decay and dental injuries associated with Suboxone’s sublingual film version.
Social Media Harm Lawsuits are being filed against social media companies for allegedly causing mental health issues in children and teens.
Claims allege that companies like Meta, Google, ByteDance, and Snap designed addictive platforms that led to anxiety, depression, and other mental health issues without adequately warning users or parents.
Transvaginal Mesh Lawsuits are being filed against manufacturers of transvaginal mesh products used to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
Claims allege that companies like Ethicon, C.R. Bard, and Boston Scientific failed to adequately warn about potential dangers — including erosion, pain, and infection.
Bair Hugger Warming Blanket Lawsuits involve claims against 3M — alleging their surgical warming blankets caused severe infections and complications (particularly in hip and knee replacement surgeries).
Plaintiffs claim 3M failed to warn about potential risks — despite knowing about increased risk of deep joint infections since 2011.
Baby Formula NEC Lawsuit claims are being filed against manufacturers of cow’s milk-based baby formula products.
Claims allege that companies like Abbott Laboratories (Similac) and Mead Johnson & Company (Enfamil) failed to warn about the increased risk of necrotizing enterocolitis (NEC) in premature infants.
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?