Depo Provera lawsuits claim that the pharmaceutical manufacturer failed to warn patients about the risk of developing meningiomas, benign brain tumors that can require invasive surgery and cause life-altering symptoms.
The foundation for these pharmaceutical product liability cases emerged from groundbreaking research published in March 2024, revealing that women who used Depo-Provera for more than a year faced a 5.6-fold increased risk of developing these brain tumors.

These lawsuits seek compensation for medical expenses, surgical costs, lost wages, pain and suffering, and the profound impact these tumors have on patients’ quality of life.
The FDA has approved Depo-Provera since 1992 as an injectable contraceptive, yet current U.S. labeling contains no warning about meningioma risks despite mounting evidence.
Medical Conditions Linked to Depo Provera Claims
Recent scientific evidence has established a compelling connection between prolonged Depo Provera usage and the development of meningiomas, tumors that form in the protective layers surrounding the brain and spinal cord.
The pivotal March 2024 study published in the British Medical Journal examined over 108,000 women in France over a decade, finding that injection exposure to the synthetic hormone medroxyprogesterone acetate was associated with a 53% increase in meningioma risk.
Research shows that approximately 70% of meningiomas express progesterone receptors, which may explain how the synthetic hormone (progestin) in Depo-Provera stimulates tumor growth.
These findings have prompted thousands of women to seek legal recourse for their previously unexplained brain tumors.
The primary medical conditions and symptoms associated with Depo Provera meningiomas typically involve:
- Meningiomas of varying sizes and locations: Including convexity meningiomas, parasagittal meningiomas, and skull base meningiomas
- Neurological symptoms: Severe headaches, vision impairment, double or blurry vision, and hearing loss
- Cognitive impacts: Memory loss, mental changes, difficulty concentrating, and language difficulties
- Physical manifestations: Seizures, arm or leg weakness, balance problems, and facial numbness
- Secondary complications: Tinnitus, personality changes, and long-term disability following surgical intervention
Despite multiple labeling changes, with the most recent occurring in July 2024, the U.S. version of the Depo Provera warning label still contains no mention about the established link between brain tumor development in individuals receiving Depo Provera injections.
This absence of vital safety information forms the cornerstone of failure-to-warn claims, as patients were unable to make informed decisions about their contraceptive choices while unknowingly accepting a substantially elevated risk of developing brain tumors requiring advanced neurosurgical intervention.
Types of Damages Recoverable in Birth Control Injury Cases
Pharmaceutical injury cases involving Depo-Provera meningiomas encompass multiple categories of compensable damages that reflect both the immediate and long-term impacts of brain tumor diagnosis and treatment.
Economic damages form the foundation of most claims, covering quantifiable losses such as neurosurgery costs, ongoing medical monitoring, lost wages during recovery, and diminished earning capacity.
Non-economic damages address the profound personal toll, including chronic pain, emotional distress, loss of enjoyment of life, and the psychological trauma of undergoing brain surgery.
The comprehensive categories of damages available in Depo-Provera brain tumor cases include, but are not limited to:
- Medical expenses: Past and future costs for brain imaging (MRI/CT scans), neurosurgical procedures, hospital stays, and follow-up care
- Lost income: Wages lost during treatment and recovery, plus future earning capacity if cognitive or physical impairments persist
- Pain and suffering: Physical pain from the tumor and surgical intervention, plus emotional distress from diagnosis and treatment
- Loss of consortium: Impact on marital relationships and family dynamics due to personality changes or physical limitations
- Punitive damages: Potential awards to punish Pfizer for allegedly concealing known risks, though availability varies by state
Documentation requirements for optimal compensation include comprehensive medical records showing tumor diagnosis, surgical reports detailing craniotomy procedures, neurological assessments documenting functional impairments, and employment records demonstrating income loss.
For women experiencing permanent cognitive deficits or requiring lifetime medical monitoring, expert testimony from neurologists and vocational rehabilitation specialists becomes vital to establish the full scope of future care costs and economic losses associated with their meningioma diagnosis.
If you or a loved one developed a meningioma brain tumor after receiving Depo Provera prescriptions, you may be eligible to seek compensation.
Contact TruLaw using the chat on this page to receive an instant case evaluation and determine whether you qualify to join others in filing a Depo Provera shot lawsuit today.
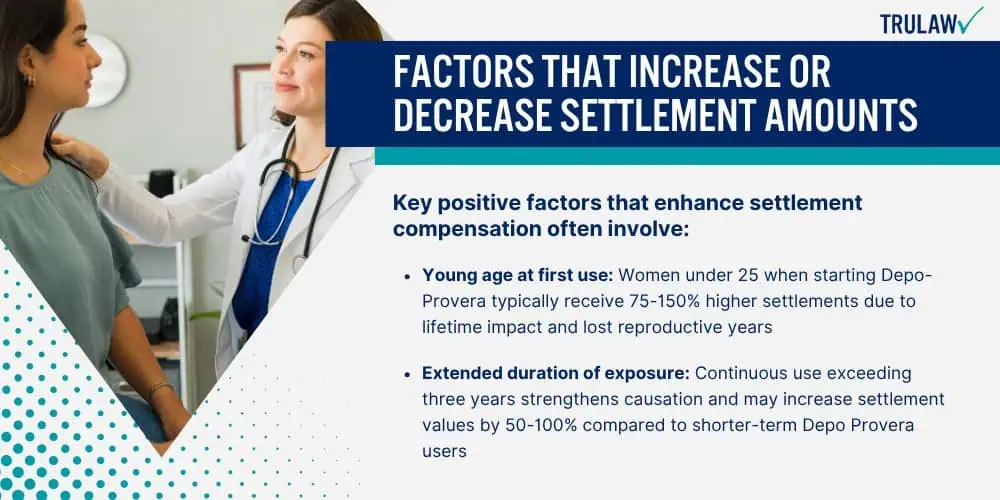
How Product Liability Laws Impact Settlement Values
Product liability laws provide multiple legal theories under which Depo Provera victims can pursue compensation, with failure-to-warn claims representing the strongest basis for recovery given Pfizer’s alleged knowledge of brain tumor risks without adequate disclosure.
Under strict liability principles, pharmaceutical manufacturers can be held responsible for injuries caused by their products regardless of intent or negligence, focusing instead on whether the product was unreasonably dangerous when used as intended.
Design defect theories may also apply, arguing that safer alternative contraceptives existed without the elevated meningioma risk.
Key legal theories that strengthen Depo-Provera settlement values typically involve:
- Failure to warn: Pfizer failed to adequately disclose meningioma risks from international studies while maintaining inadequate U.S. labeling
- Strict liability: The inherently dangerous nature of a contraceptive causing brain tumors when safer alternatives exist
- Negligence: Breach of duty to conduct adequate safety studies and promptly update warnings based on emerging evidence
- Fraudulent concealment: Potential claims that Pfizer actively withheld safety data from regulatory agencies and consumers
- State law variations: Enhanced protections in consumer-friendly jurisdictions that may allow for broader damages or longer filing deadlines
Corporate knowledge plays a pivotal role in settlement negotiations, particularly evidence suggesting Pfizer was aware of international warnings about meningioma risks while the U.S. labeling remained unchanged.
The company’s internal documents, safety committee deliberations, and communications with foreign regulatory agencies will likely influence settlement values substantially.
Precedent cases involving other hormonal medications linked to serious health risks provide a framework for valuation, with juries historically awarding substantial compensation when manufacturers prioritized profits over patient safety by delaying vital warnings about life-threatening risks.
Pfizer has faced previous pharmaceutical litigation, including a record $2.3 billion settlement with the Department of Justice for health care fraud charges, demonstrating the company’s history of regulatory violations.