Vaginal Rejuvenation Lawsuit
- Last Updated: June 12th, 2025

Attorney Jessica Paluch-Hoerman, founder of TruLaw, has over 28 years of experience as a personal injury and mass tort attorney, and previously worked as an international tax attorney at Deloitte. Jessie collaborates with attorneys nationwide — enabling her to share reliable, up-to-date legal information with our readers.
Legally Reviewed
This article has been written and reviewed for legal accuracy and clarity by the team of writers and legal experts at TruLaw and is as accurate as possible. This content should not be taken as legal advice from an attorney. If you would like to learn more about our owner and experienced injury lawyer, Jessie Paluch, you can do so here.
Fact-Checked
TruLaw does everything possible to make sure the information in this article is up to date and accurate. If you need specific legal advice about your case, contact us by using the chat on the bottom of this page. This article should not be taken as advice from an attorney.
Deceptive Health Claims, Significant Risks
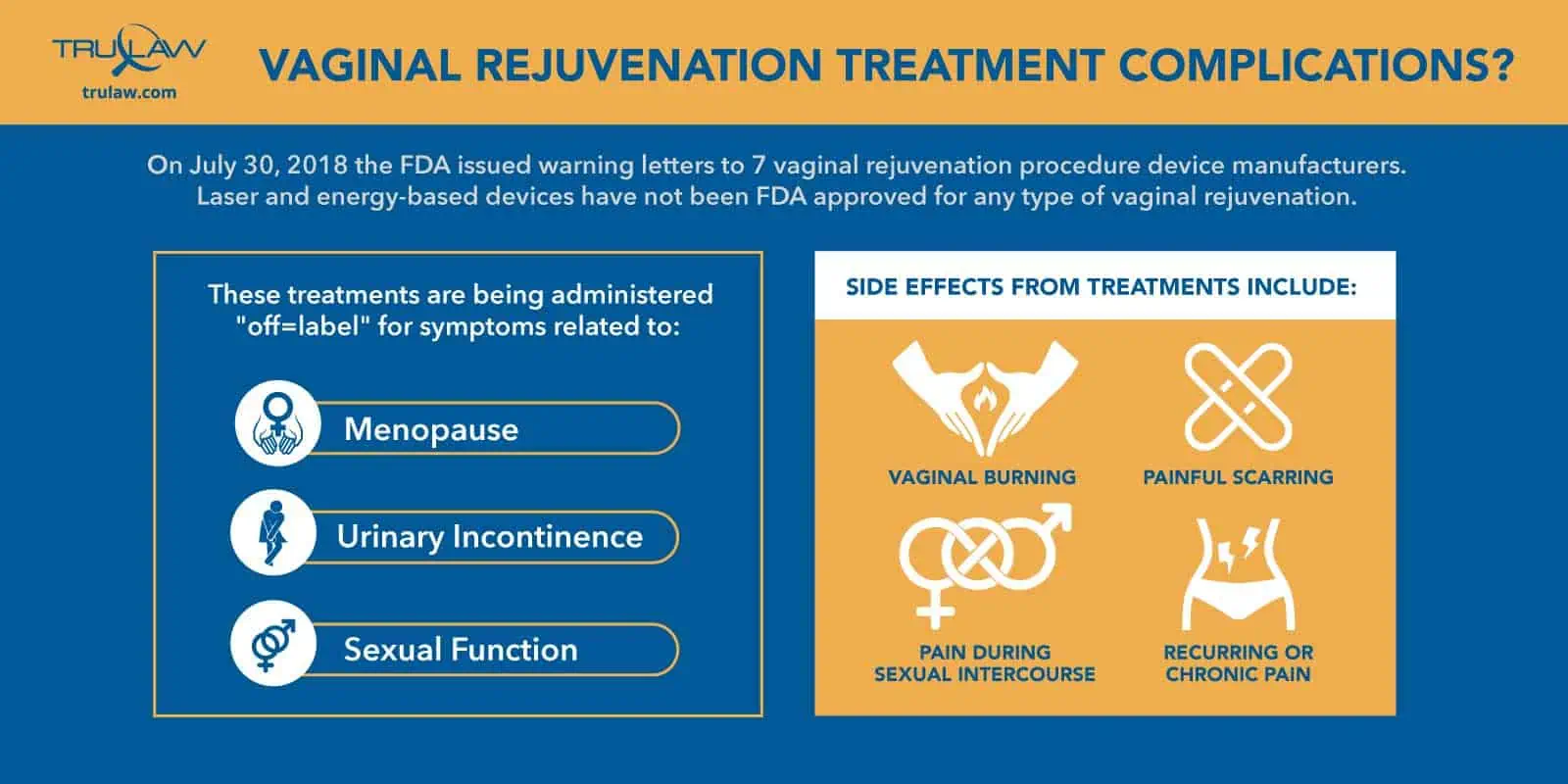
On July 30, 2018, the FDA issued a safety communication for laser and other energy-based vaginal rejuvenation treatments due to the serious risks they carry.
Women have experienced vaginal burning, serious scarring, and chronic pain.
If you have suffered from an adverse event after undergoing a rejuvenation laser treatment, you may have the right to file a vaginal rejuvenation lawsuit.

These dangerous treatments are deceptively being advertised as a way to help with vaginal looseness, urinary incontinence, and pain during intercourse.
These rejuvenation treatments are being carried out by spas, cosmetic facilities, gynecologists, and obstetricians.
However, there are no conclusive studies to suggest that these procedures can do what is being promised.
In all of the published trials to date, only several hundred women have been studied and most of these studies last only 12 weeks.
No long-term safety information is available for women.
Table of Contents
What is Vaginal Rejuvenation?
Vaginal rejuvenation is a term that covers essentially anything that alters the vaginal anatomy.
This includes both internal and external structures.
The procedure in question is one in which energy-based devices (i.e. lasers), using thermal or nonthermal energy, aim to correct and restore the optimal structure and tightness of the vagina and surrounding tissues by inducing contraction of collagen and elastin.
These are outpatient procedures, do not require anesthesia, and take 15 – 30 minutes.
There are generally three treatments spaced 6 weeks apart.
There is a more invasive rejuvenation procedure known as vaginoplasty or posterior colporrhaphy, where the separate muscles of the vagina are brought together and the extra mucosa skin from the back side is removed.
If there is external skin protruding, this can be removed so the vagina looks better aesthetically.
This procedure does require either local or general anesthesia.
Dr. Adeeti Gupta, a board-certified gynecologist based in New York, has talked about the procedures and said:
“These don’t actually make your vagina ‘tighter’ per se. Instead, the laser procedure causes your below-the-belt tissue to become inflamed, creating scar tissue. This can look like a tightening of the vaginal canal.”
Vaginal Rejuvenation Therapy Not FDA Approved
Rejuvenation procedures are becoming more and more popular among young women, especially after childbirth, but, to date, laser and other energy-based devices have not been FDA approved for any type of vaginal rejuvenation, menopause symptoms, or sexual function.
The FDA is specifically looking at procedures that use CO2 lasers and other energy-based devices used to destroy or reshape vaginal tissue.
In regards to their investigation, the FDA commissioner Dr. Scott Gottlieb said:
“These products carry serious risks and don’t have adequate evidence to support their use for these purposes. We are deeply concerned women are being harmed.”
These devices have only been approved for the treatment of abnormal or pre-cancerous cervical or vaginal tissue, genital warts, and hysterectomies.
Vaginal Rejuvenation Side Effects
Vaginal Rejuvenation side effects include, but are not limited to:
- Vaginal burning
- Painful scarring
- Bleeding
- Pain during sexual intercourse
- Recurring or chronic pain
- Loss of sensation, numbness
- Swelling
Women may also experience nerve damage after undergoing a treatment, and because it is very hard for nerves to regenerate, this side effect may be permanent.
The full extent of the risks these procedures carry is still unknown, but there have been at least 14 reports of adverse events.
If you, or anyone you know, has experienced side effects after a vaginal rejuvenation treatment, we urge you to report the adverse event to the FDA.
7 companies Issued FDA Warning Letter
These companies were warned because of their “inappropriate marketing” for vaginal rejuvenation treatments:
- Alma Lasers (FemiLift, Pixel CO2 Laser System)
- BTL Industries, Inc (Exilis Ultra 360 System)
- Cynosure (MonaLisa Touch, DEKA Smart Xide Laser System)
- Inmode (FormaV and FractoraV lasers)
- Sciton (JOULE Multi-Platform System)
- Thermigen (THERMIva)
- BTL Aesthetics (Venus Fiore RF Ablation System)
The letters sent are requesting that these manufacturers address these serious concerns regarding vaginal rejuvenation treatments within 30 days.
If the concerns are not addressed, the FDA will consider potential enforcement actions next.
Can I File A Vaginal Rejuvenation Lawsuit?
TruLaw is looking into vaginal rejuvenation lawsuits now.
If you have suffered from any side effects after having a laser vaginal rejuvenation treatment, please fill out the Contact Us form on this page so we can discuss your legal options.
We will also make sure to keep you updated on the activity of the FDA actions as well as the status of these cases.

Managing Attorney & Owner
With over 25 years of legal experience, Jessica Paluch-Hoerman is an Illinois lawyer, a CPA, and a mother of three. She spent the first decade of her career working as an international tax attorney at Deloitte.
In 2009, Jessie co-founded her own law firm with her husband – which has scaled to over 30 employees since its conception.
In 2016, Jessie founded TruLaw, which allows her to collaborate with attorneys and legal experts across the United States on a daily basis. This hypervaluable network of experts is what enables her to share the most reliable, accurate, and up-to-date legal information with our readers!
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?
At TruLaw, we fiercely combat corporations that endanger individuals’ well-being. If you’ve suffered injuries and believe these well-funded entities should be held accountable, we’re here for you.
With TruLaw, you gain access to successful and seasoned lawyers who maximize your chances of success. Our lawyers invest in you—they do not receive a dime until your lawsuit reaches a successful resolution!
AFFF Lawsuit claims are being filed against manufacturers of aqueous film-forming foam (AFFF), commonly used in firefighting.
Claims allege that companies such as 3M, DuPont, and Tyco Fire Products failed to adequately warn users about the potential dangers of AFFF exposure — including increased risks of various cancers and diseases.
Depo Provera Lawsuit claims are being filed by individuals who allege they developed meningioma (a type of brain tumor) after receiving Depo-Provera birth control injections.
A 2024 study found that women using Depo-Provera for at least 1 year are five times more likely to develop meningioma brain tumors compared to those not using the drug.
Suboxone Tooth Decay Lawsuit claims are being filed against Indivior, the manufacturer of Suboxone, a medication used to treat opioid addiction.
Claims allege that Indivior failed to adequately warn users about the potential dangers of severe tooth decay and dental injuries associated with Suboxone’s sublingual film version.
Social Media Harm Lawsuits are being filed against social media companies for allegedly causing mental health issues in children and teens.
Claims allege that companies like Meta, Google, ByteDance, and Snap designed addictive platforms that led to anxiety, depression, and other mental health issues without adequately warning users or parents.
Transvaginal Mesh Lawsuits are being filed against manufacturers of transvaginal mesh products used to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
Claims allege that companies like Ethicon, C.R. Bard, and Boston Scientific failed to adequately warn about potential dangers — including erosion, pain, and infection.
Bair Hugger Warming Blanket Lawsuits involve claims against 3M — alleging their surgical warming blankets caused severe infections and complications (particularly in hip and knee replacement surgeries).
Plaintiffs claim 3M failed to warn about potential risks — despite knowing about increased risk of deep joint infections since 2011.
Baby Formula NEC Lawsuit claims are being filed against manufacturers of cow’s milk-based baby formula products.
Claims allege that companies like Abbott Laboratories (Similac) and Mead Johnson & Company (Enfamil) failed to warn about the increased risk of necrotizing enterocolitis (NEC) in premature infants.
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?