Power Morcellator Lawsuits | Linked to Uterine Cancer
- Last Updated: June 12th, 2025

Attorney Jessica Paluch-Hoerman, founder of TruLaw, has over 28 years of experience as a personal injury and mass tort attorney, and previously worked as an international tax attorney at Deloitte. Jessie collaborates with attorneys nationwide — enabling her to share reliable, up-to-date legal information with our readers.
Legally Reviewed
This article has been written and reviewed for legal accuracy and clarity by the team of writers and legal experts at TruLaw and is as accurate as possible. This content should not be taken as legal advice from an attorney. If you would like to learn more about our owner and experienced injury lawyer, Jessie Paluch, you can do so here.
Fact-Checked
TruLaw does everything possible to make sure the information in this article is up to date and accurate. If you need specific legal advice about your case, contact us by using the chat on the bottom of this page. This article should not be taken as advice from an attorney.
Intro to the Power Morcellator Lawsuits

Morcellation devices put women at increased risk of uterine cancer when used during hysterectomy or myomectomy procedures.
Women suffering from unexpected cancers are filing lawsuits.
Table of Contents
Power Morcellator Linked to Uterine Cancer
Laparoscopic power morcellators are tools used in fibroid removal procedures that cut up uterine growth so that the growth can be more easily removed using noninvasive procedures.
They could be used as part of either a myomectomy (removal of uterine fibroids) or hysterectomy (removal of uterus).
The power morcellator essentially cuts uterine growth (fibroids) into very small pieces that can be removed by vacuum through a small incision.
Power Morcellator Lawsuits Filed
Numerous women have filed morcellator lawsuits against Johnson & Johnson and other manufacturers of these morcellation devices, alleging they were not informed of the risks before undergoing their laparoscopic procedures.
In order to streamline early trial proceedings and make the process more convenient and efficient for all parties involved, federal court cases involving Ethicon power morcellators were coordinated into multidistrict litigation (MDL) in Kansas.
The MDL is used to determine pretrial rulings in cases that involve many plaintiffs but a common question of fact.
Cases currently pending in the MDL will be transferred back to their originating court for trial if settlement does not occur prior to trial.
Women who believe that their cancer diagnosis was made worse as a result of their choice to undergo a less invasive fibroid surgery or hysterectomy are currently filing lawsuits.
FDA Warning
In April 2014, the U.S. Food and Drug Administration (FDA) issued a safety communication to warn physicians and patients that power morcellators could spread undetected cancer cells.
Because there is no definitive way to know for sure if cancer cells are present prior to a procedure, the FDA recommended at the time that patients be counseled on the risks associated with power morcellators.
The agency also advised that morcellators should not be used in the majority of hysterectomies and myomectomies, since vaginal procedures are typically associated with better results and fewer complications.
Johnson & Johnson Recalls Power Morcellators
As a result of a public outcry and the FDA warning, the largest manufacturer of the Power Morcellator tool, Johnson & Johnson, pulled the device off the market but there are still women today learning of the connection between a laparoscopic procedure and their uterine cancer diagnosis.
Power Morcellator Used in Large Numbers Prior to Recall
Many women choose to undergo laparoscopic hysterectomy or myomectomy because it is associated with benefits such as a shorter post-operative recovery time and reduced risk of infection compared to abdominal hysterectomy and myomectomy.
The minimally invasive nature of surgery involving a power morcellator made it an appealing choice for many women undergoing hysterectomies or myomectomies until Johnson & Johnson pulled the device from the market.
According to investigations, Approximately 1 in 350 women undergoing these types of surgeries have an undetected form of uterine cancer.
In these women, there is a risk that the morcellator will spread the cancerous tissue, decreasing the likelihood of long-term survival.
After public outcry and pressure from medical professionals, the FDA issued a strong warning regarding the use of power morcellators, in which it discouraged their use and directed surgeons to discuss the risks of using power morcellators with their patients.
Frequently Asked Questions
-
Sometimes you need a lawyer near you and sometimes its best to hire a lawyer based on the lawyer’s resources and experiences.
The right lawyer for mass-tort litigations may not be your local lawyer.
Mass tort cases filed all over the country are often consolidated into a single courtroom in order to move the many lawsuits through the courts in the most effective and efficient way.
A lawyer experienced with the multidistrict litigation process with the ability to represent clients in all 50 states, is likely to be a good fit for mass tort litigation.
TruLaw is not afraid to take on the largest drug and medical device companies in the world.
We work with trusted legal affiliates to make sure that TruLaw clients have the resources and experiences needed to hold big business accountable when they put profits over people.
-
Your Power Morcellator Surgery lawsuit is designed to help you financially recover from injuries that were caused by someone else.
We hope putting your trust in TruLaw will take away your concern of protecting your legal rights., but it is most important to us that you spend your time recovering physically.
Your lawsuit should assist in covering your medical bills, the amount of income and benefits that you lost as a result of your injury and, if your injuries are permanent, we will look to recover for your permanent disfigurement.
In addition, it is always our hope that your lawsuit will help us to remove dangerous drugs, toxins and devices from the market.
We are not only lawyers, but also safety advocates that believe in getting information out to the public so no more people are injured. We hope you will join us in the role as a safety advocate.
-
We often hear injured people refer to their personal injury case as a “class action” because their case was grouped together in a lawsuit with other injured people.
This is most often NOT the case.
Often, individual cases are grouped together so the attorneys and judge can address common procedural issues initially, saving time for the injured parties and the court, but this is very generally referred to as a “mass tort.”
A Mass tort refers to civil actions involving numerous plaintiffs against one or a few corporate defendants in state or federal court.
Class actions are mass torts that are generally used on financial losses and multidistrict litigations (MDL) are generally used on personal injury claims, often in product liability cases.
MDL is a procedural tool used when plaintiffs have incurred injuries from products manufactured by the same defendant(s).
Even when plaintiffs incur injuries from the same defendant(s), the amount of damages they may recover for those injuries are often substantially different from other plaintiffs included in the same lawsuit.
It is important to understand that mass tort cases are an effective tool to getting the attention of the large drug and device companies.
MDLs assist lawyers in determining exactly what the drug and device companies knew about the risks their products caused and whether or not they should have warned consumers.
Too often, consumers believe that they can file a single lawsuit and get the attention of big drug companies.
This is very hard to do.
Technically, MDLs do not happen until a judicial panel transfers individual cases to a single court.
Depending on when your lawsuit is filed, you may find yourself automatically transferred to the MDL court or you may wait to learn when and if the JPML believes an MDL is the proper venue for the mass tort.
But, rest assured, even if your case is included in an MDL, TruLaw lawyers will treat your injuries, your medical history and your financial needs separately.
We are aware that not all cases are the same.
-
We understand the frustration in waiting to hear about settlements in product liability lawsuits.
Unfortunately, in drug and device cases, we have no choice but to sue some of the most profitable companies in the world.
Big Pharma has deep pockets and lawsuits are a cost of doing business for them.
They are not inclined to settle until it makes business sense to them.
TruLaw lawyers building our cases with an eye on winning in court as well as settlement, when we believe that is the best result for our clients.
We will never settle without advising you of your options, and we will keep you posted on our progress, to the extent we are legally able.
-
A corporation, by definition is profit seeking.
There is no requirement that a corporation act morally.
Unfortunately, too often we see dangerous drugs, devices and products remain on the market when corporations prioritize profit over people.
If these same corporations warn consumers of these risks, there is no case.
We only pursue lawsuits on behalf of individuals who were not warned of the risk associated with the dangerous drug, device or product on the market.
TruLaw is pursuing Power Morcellator Lawsuits because we believe consumers were not properly warned of the risks of injury.
-
Did a recent Power Morcellator commercial grab your attention?
Did you find our site because you were wondering if you qualify for Power Morcellation Surgery lawsuits?
We built the Power Morcellator Instant Case Evaluator ℠ as a no cost/no obligation place for you to find answers about your legal rights.
If you found us today, you are looking for instant answers to whether you should file a Power Morcellator Lawsuit and we want to help you.
We believe that in order for you to make important decisions about your health and your legal rights, you need to start with information.
We provide you this valuable information so you are prepared to talk to a lawyer.

Managing Attorney & Owner
With over 25 years of legal experience, Jessica Paluch-Hoerman is an Illinois lawyer, a CPA, and a mother of three. She spent the first decade of her career working as an international tax attorney at Deloitte.
In 2009, Jessie co-founded her own law firm with her husband – which has scaled to over 30 employees since its conception.
In 2016, Jessie founded TruLaw, which allows her to collaborate with attorneys and legal experts across the United States on a daily basis. This hypervaluable network of experts is what enables her to share the most reliable, accurate, and up-to-date legal information with our readers!
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?
At TruLaw, we fiercely combat corporations that endanger individuals’ well-being. If you’ve suffered injuries and believe these well-funded entities should be held accountable, we’re here for you.
With TruLaw, you gain access to successful and seasoned lawyers who maximize your chances of success. Our lawyers invest in you—they do not receive a dime until your lawsuit reaches a successful resolution!
AFFF Lawsuit claims are being filed against manufacturers of aqueous film-forming foam (AFFF), commonly used in firefighting.
Claims allege that companies such as 3M, DuPont, and Tyco Fire Products failed to adequately warn users about the potential dangers of AFFF exposure — including increased risks of various cancers and diseases.
Depo Provera Lawsuit claims are being filed by individuals who allege they developed meningioma (a type of brain tumor) after receiving Depo-Provera birth control injections.
A 2024 study found that women using Depo-Provera for at least 1 year are five times more likely to develop meningioma brain tumors compared to those not using the drug.
Suboxone Tooth Decay Lawsuit claims are being filed against Indivior, the manufacturer of Suboxone, a medication used to treat opioid addiction.
Claims allege that Indivior failed to adequately warn users about the potential dangers of severe tooth decay and dental injuries associated with Suboxone’s sublingual film version.
Social Media Harm Lawsuits are being filed against social media companies for allegedly causing mental health issues in children and teens.
Claims allege that companies like Meta, Google, ByteDance, and Snap designed addictive platforms that led to anxiety, depression, and other mental health issues without adequately warning users or parents.
Transvaginal Mesh Lawsuits are being filed against manufacturers of transvaginal mesh products used to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
Claims allege that companies like Ethicon, C.R. Bard, and Boston Scientific failed to adequately warn about potential dangers — including erosion, pain, and infection.
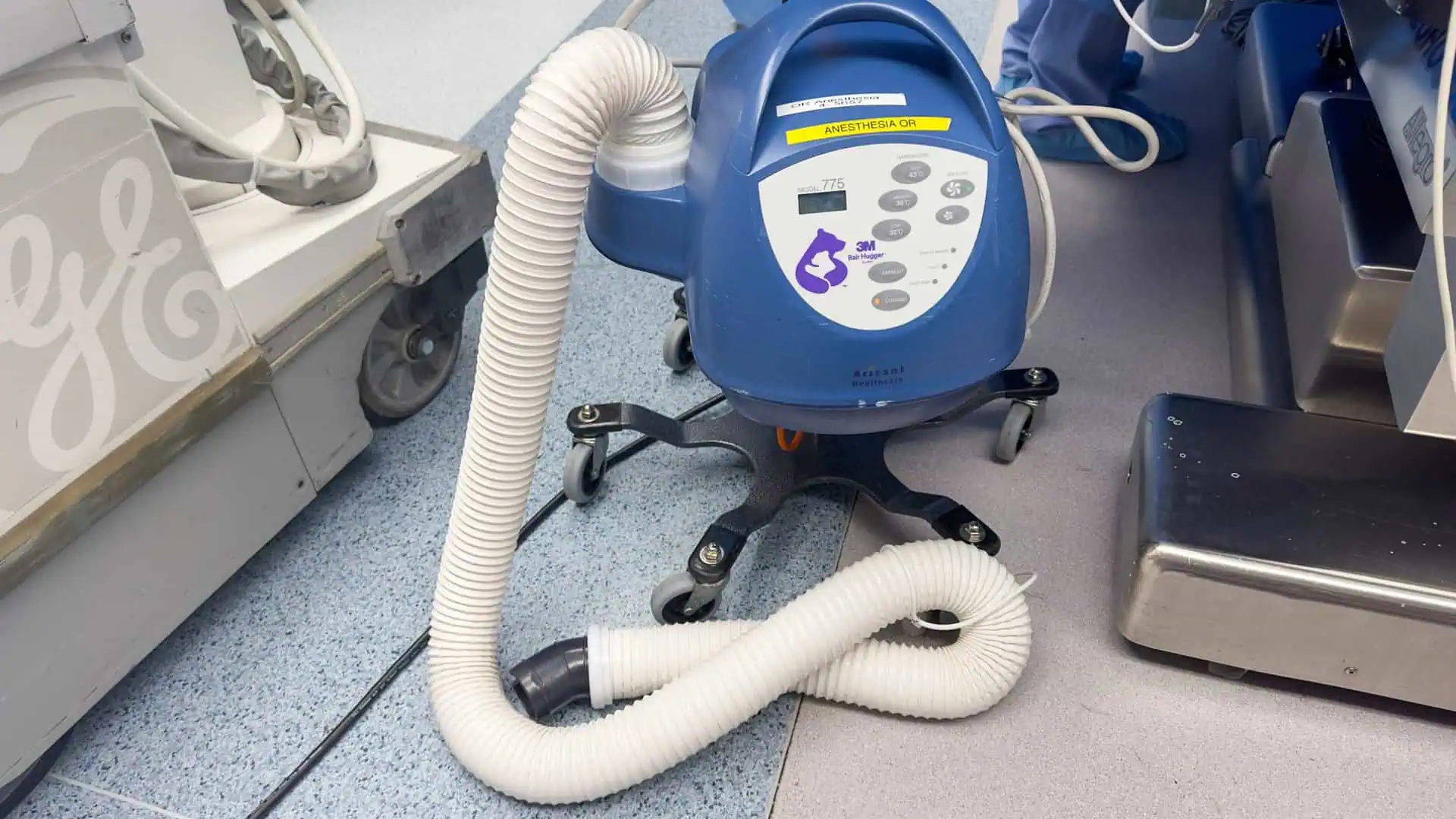
Bair Hugger Warming Blanket Lawsuits involve claims against 3M — alleging their surgical warming blankets caused severe infections and complications (particularly in hip and knee replacement surgeries).
Plaintiffs claim 3M failed to warn about potential risks — despite knowing about increased risk of deep joint infections since 2011.
Baby Formula NEC Lawsuit claims are being filed against manufacturers of cow’s milk-based baby formula products.
Claims allege that companies like Abbott Laboratories (Similac) and Mead Johnson & Company (Enfamil) failed to warn about the increased risk of necrotizing enterocolitis (NEC) in premature infants.
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?