Essure Lawsuits | Essure Birth Control Device Litigation
- Last Updated: June 12th, 2025

Attorney Jessica Paluch-Hoerman, founder of TruLaw, has over 28 years of experience as a personal injury and mass tort attorney, and previously worked as an international tax attorney at Deloitte. Jessie collaborates with attorneys nationwide — enabling her to share reliable, up-to-date legal information with our readers.
Legally Reviewed
This article has been written and reviewed for legal accuracy and clarity by the team of writers and legal experts at TruLaw and is as accurate as possible. This content should not be taken as legal advice from an attorney. If you would like to learn more about our owner and experienced injury lawyer, Jessie Paluch, you can do so here.
Fact-Checked
TruLaw does everything possible to make sure the information in this article is up to date and accurate. If you need specific legal advice about your case, contact us by using the chat on the bottom of this page. This article should not be taken as advice from an attorney.
Essure Lawsuits: July 20th, 2018 Update
Bayer discontinues the sale of Essure and will stop distributing the device in the United States after December 31, 2018.
According to regulatory filings, more than 16,800 Essure lawsuits have been filed against Bayer, the manufacturer of Essure, by women who have experienced life-altering injuries who claim that Bayer did not fully disclose the risk of injury from Essure, a non-surgical permanent birth control IUD.
The number of these contraceptive lawsuits may continue to rise after the problems with the device were highlighted in the July 2018 Netflix documentary called “The Bleeding Edge” which covered serious issues with the medical device industry in the United States.

Many women who have been injured by the device have come together to share their stories in the Facebook group called Essure Problems which has over 37,000 members.
This group allows women to be able to talk to each other about the devastation Essure has caused in their lives and helps them feel less alone in their ongoing struggle.
Essure has been removed from the market in many countries, and the U.S. is going to follow suit by the end of 2018.
Table of Contents
Lawsuit Updates
-
January 2016 Updates:
March 1st ,2016: Blackbox Warning & Checklist Required
FDA requires “black box” label warning about Essure’s serious side effects, plus a checklist for doctors to discuss potential risks with patients.
-
January 2015 Updates:
September 24th, 2015: FDA Investigations
FDA holds a public meeting as a result of growing reports of complications.
-
October 2013 Updates:
October 20th, 2013: Safety Label Changes
FDA review of Essure – label updated to include risks of chronic pelvic pain and device migration.
-
June 2013 Updates:
June 5th, 2013: Bayer Purchases Conceptus
Bayer Group purchases 96.4% of Conceptus, Inc. for $1.1 billion.
Conceptus owned Essure which had been used by more thatn 750,000 women around the globe by this time.
-
January 2011 Updates:
July 1st, 2011: Safety Label Changes
Physician label updated to remove contraindication and to revise a warning regarding nickel sensitivity in physicians label.
-
January 2002 Updates:
November 4th, 2002: Essure Approval
Essure FDA approved as a Class III Medical Device.
-
January 1990 Updates:
52 deaths attributed to Bayer’s statin drug, Baycol.
Baycol was discontinued in 2011.
-
January 1983 Updates:
Cutter’s Factor VII, hemophilia drug, was found to be the source of the high rate of hemophiliacs with AIDS (Cutter was purchased by Bayer in 1978).
-
January 1960 Updates:
Bayer introduced a pregnancy test, Primodos, that consisted of 2 pills that was later blamed for birth defects – it was removed from the market in the 1970’s.
The Truth In Essure?
According to Bayer, Essure is a “non-surgical” permanent birth control solution for women:
- Women who are implanted with Essure are 10 times more likely to need additional surgeries than women who get standard sterility surgery.
- Studies suggest that as many as 9.6% of women could become pregnant after an Essure implant – that is more than four times the estimated risk after a traditional sterility surgery.
- The truth is Bayer Figured Out How to Get the Device In… But they Never Figured Out How to Get it Out!
What Is Essure?
Essure is a widely used medical device marketed as a permanent birth control for women ages 21 to 45.
Approved by the U.S. Food and Drug Administration in 2002, Essure is a soft, flexible metal spring made of two nickel-titanium and stainless steel coils with a filler made of PET (polyethylene terephthalate) fibers.
Essure is the only permanent birth control that involves a non-surgical procedure, but it does not protect against HIV or other sexually transmitted diseases.
The device is typically inserted in an OB-GYN’s office in ten minutes or less, without incisions or general anesthesia.
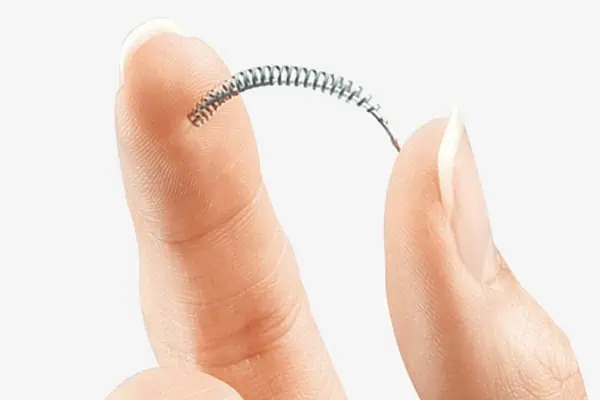
Historically, Essure was marketed as a very effective method of birth control with a success rate estimated at 99.83%.
However, a study published in the journal Contraception concluded that as many as 9.6% of women could become pregnant within 10 years of having the Essure device implanted.
This is nearly four times the estimated risk after a laparoscopic tubal ligation.
More than 1 million women in the U.S. have had the device implanted.
How Does it Work?
Essure is inserted in an OB-GYN’s office in ten minutes or less, according to Essure implant guidance.
Removal is generally not discussed, and yet many women will experience complications leading to the removal of the Essure device.
One Essure coil is inserted through the vagina and cervix into each fallopian tube.
Over the next several months, scar tissue forms around the coils, creating a barrier that blocks the tubes and keeps the eggs from being fertilized or making their way to the uterus, thus preventing conception.
Women implanted with Essure are required to return three months after the procedure for a follow-up X-ray utilizing dye to confirm that the tubes are fully blocked.
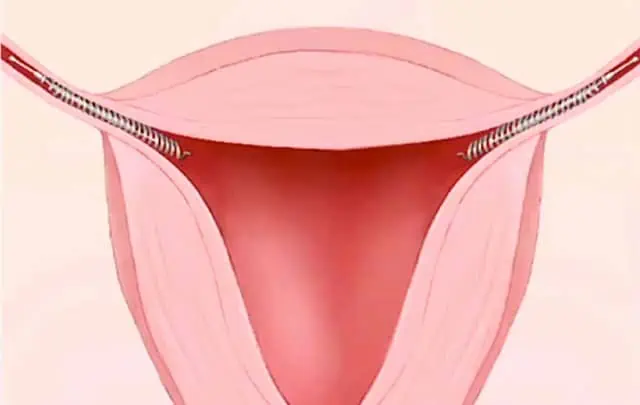
Removing the Essure device has been a source of much frustration for women experiencing complications from the Essure device.
There are very few doctors trained to remove the devices – Bayer has not trained doctors in removal.
As more and more women seek removal, several OB-GYNs have emerged as specialists in Essure removal.
Women seeking removal are often guided to these specialists through word of mouth and social media support groups.
Removal of the Essure device intact is very important so that parts of the nickel and steel coils do not remain in the women’s body opening it up to future complications.
To do this, the majority of women (75.2% according to a recent online survey of 564 women) require a hysterectomy, with an alarming 44% of these women requiring more than one surgery.
Essure Lawsuits Filed For Complications From Essure
Thousands of Essure lawsuits have been filed against Bayer Inc. (the manufacturer of Essure) by women who have experienced life-changing complications.
Migration/Expulsion of Device
Women report migration of the Essure device outside the uterus and can the implant can get lodged in organs or the abdominal wall.
Essure devices have also been found in the uterus.
Injuries resulting from migration of the Essure device vary – women have found the coils of the device in many different locations in their body, sometimes puncturing organs.
Invasive hysterectomies have been required to remove these devices. Sometimes multiple surgeries are required to remove the devices.
Painful Symptoms / Constellation of Symptoms
Women describe many symptoms that have been explained by their doctors as their bodies learning to accept the coils – some women find the pain does go away but for many women, these symptoms continue and even increase.
Some women describe a constellation of symptoms while their body fights to reject the device in their body.
These symptoms vary tremendously with the women and include bloating/weight gain, pain with sexual intercourse, incontinence, chronic pelvic pain and cramping, heavy periods and more.
Fetal Deaths Under-Reported
Congressman Mike Fitzpatrick (R-PA) recently revealed 303 fetal deaths caused by Essure, as opposed to the 5 deaths reported to the FDA.
On November 4, 2015 he announced the introduction of the E-Free Act — legislation to remove Essure from the market.
According to a private analyst who combed through the FDA’s public database, the FDA may have greatly underestimated the number of fetal deaths among women who became pregnant after using Essure.
The FDA reported a mere five fetal deaths while Madris Tomes, founder, and CEO of Device Events, noted there were 303 reports of fetal deaths tied to Essure as reported in the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database.
Ectopic Pregnancies
Women implanted with Essure have a higher percentage of Ectopic pregnancies.
An ectopic pregnancy cannot support the life of a fetus for very long and risks the health of the mother if immediate care is not sought.
Women with Essure devices have a higher percentage of ectopic pregnancies.
Ectopic pregnancies occur when fertilization of the egg happens outside of the uterus.
Ectopic pregnancies can’t proceed normally.
Nickel Injuries/Allergies
Originally, Conceptus warned women to test for nickel allergies prior to Essure implant.
But, proudly had this warning and the required skin test removed from the label in 2011.
Many women describe allergy-like reactions to Nickel that include hair loss, dental issues, weight gain, fatigue and more.
Essure is 55% nickel – Many women report side effects that may actually be reactions to nickel in their body.
Reported side effects that may be an allergic reaction to nickel include hair loss, dental issues, weight gain, chronic fatigue, and more.
The original Essure label required a skin test and warned of nickel allergies, this was removed in 2011.
Top Complications from Essure
The top complications from the Essure birth control device include, but are not limited to:
- FEARS (FDA Adverse Event Reporting System) Analysis – They tell you about Adverse Events they know of, not what’s REALLY happening to you!
- Bayer says about 1 million Essure devices have been sold worldwide. More women will be at risk for complications while the FDA continues to study this device.
NOTE: An adverse event is any undesirable experience associated with the use of a medical product in a patient.
Broken down into two (2) categories, here are the top complications from Essure:
Original Labeled – 2002
- 1 out of 7 women would have improper placement and will require a second placement procedure.
- Mild to moderate pain
- Overabsorption of fluid
- Cramping
- Vaginal bleeding
- Pelvic or back discomfort for a few days
- Nausea and/or vomiting
- Infection
- Temporary changes in menstrual cycle
- Essure device may be expelled from body
- Broken Essure micro-insert
Labeled as “rare”
- Essure device may be expelled from body
- Broken Essure micro-insert
Safety Information
A black box warning is the strictest warning put in the labeling of prescription drugs or drug products by the Food and Drug Administration (FDA) when there is reasonable evidence of an association of a serious hazard with the drug.
The black box warning must be part of the physician and patient labeling materials and includes a statement noting the risks associated with the device.
September 2, 2016 – The FDA ordered Bayer to conduct a three-year clinical study regarding the safety and efficacy of Essure.
They are also requiring Bayer to submit a study protocol within 15 months of the order.
October 31, 2016 – The FDA issued the final guidance, “Labeling for Permanent Hysteroscopically-Placed Tubal Implants Intended for Sterilization”
Essure Black Box Warning
WARNING: Some patients implanted with the Essure System for Permanent Birth Control have reported adverse events, including perforation of the uterus and/or fallopian tubes, intra-abdominal or pelvic device migration, persistent pain, and allergy or hypersensitivity reactions.
Some of these reported events resulted in device removal that required abdominal surgery.
This information should be shared with patients considering sterilization with the Essure device during a discussion of the benefits and risks of the device.
Essure Patient Decision Checklist
April 9, 2018 – In lieu of removing the device from the market, the FDA announced a new “decision checklist” that must be signed by a patient and doctor before implantable sterilization devices such as Essure can be implanted.
The checklist is supposed to list every significant adverse event and patient outcomes.
It is also supposed to contain information related to the removal and/or reversal of the device.
Each item in the checklist should be accompanied by a line for the patient to initial, acknowledging their understanding of the information.
Furthermore, the checklist should include a summary at the end of the document.
This should be followed by signature lines for both the patient and physician.
A copy of the Patient Decision Checklist will be given to the patient and a copy will also be provided to the patient.
What Is A Patient Decision Checklist?
According to the FDA, a patient checklist highlights the key risk and benefit information and will be included at the end of the patient-labeling brochure.
The checklist is intended to be reviewed and signed by the patient and physician and should be printed in a fashion where it can be easily separated from the remainder of the patient information brochure.
A History Of Problems For Bayer Results In Essure Lawsuits
Bayer AG was founded in 1863 by Friedrich Bayer and Johann Friedrich Weskott.
In 1925, Bayer became part of IG Farben, A German chemical company conglomerate that engaged in human experimentation on Auschwitz prisoners during World War II.
After World War II, the allies broke the companies up and Bayer continued to operate as its own drug company.
Marketed as “Science for a Better Life” Bayer has been involved with a number of drugs and devices that have notably had legal issues.

Essure was initially manufactured by Conceptus, a company that began focusing on the design, development, and clinical testing of the permanent form of birth control in 1998.
Essure had shown promise by eliminating the cutting, clipping, and burning associated with tubal ligation.
After clinical testing, Conceptus began marketing Essure commercially in Australia, Singapore, Europe, and Canada.
Bayer acquired Conceptus Inc. for about $1.1 billion in 2013 to add a permanent contraception device (Essure) to complement the German company’s offerings in women’s health.
Essure was approved by FDA in 2002.
When the U.S. Food and Drug Administration (FDA) approved Essure in 2002, the agency required the original manufacturer, Conceptus, to monitor the outcomes of women who were enrolled in the five-year clinical trial upon which the agency’s approval was based.
The extended approval time was for an additional four years.
In a May 1, 2015 news release, Bayer released the post-market report on the safety, effectiveness, and tolerability of Essure, a report experts say is one-sided because it:
- Never mentions that nearly one-third of the women originally enrolled in the study dropped out for various reasons.
- Understates the levels of pelvic pain reported by women who did complete the study.
- Does not disclose that the study’s lead author is a paid consultant to Bayer.
Further, according to a Health News Review report, Conceptus failed to report the outcome of the five-year study and the results were submitted for publication a year after Bayer acquired the device.
Bayer continues to market Essure as one the most effective, affordable and convenient means of birth control – ideal for the busy 21st-century woman and mother.
Company officials say it is sold in at least 23 countries, and at least one million devices are in use worldwide:
- The Truth is, Women who are implanted with Essure are 10 times more likely to need new additional operations as women who get standard sterility surgery. And yet, Bayer continues to market their Essure implant as “non-surgical”.
- Bayer Profits From More than 8,000 Essure Implants Each Year While Injured E-Sisters Fight to Have the Device Removed From the Market

Managing Attorney & Owner
With over 25 years of legal experience, Jessica Paluch-Hoerman is an Illinois lawyer, a CPA, and a mother of three. She spent the first decade of her career working as an international tax attorney at Deloitte.
In 2009, Jessie co-founded her own law firm with her husband – which has scaled to over 30 employees since its conception.
In 2016, Jessie founded TruLaw, which allows her to collaborate with attorneys and legal experts across the United States on a daily basis. This hypervaluable network of experts is what enables her to share the most reliable, accurate, and up-to-date legal information with our readers!
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?
At TruLaw, we fiercely combat corporations that endanger individuals’ well-being. If you’ve suffered injuries and believe these well-funded entities should be held accountable, we’re here for you.
With TruLaw, you gain access to successful and seasoned lawyers who maximize your chances of success. Our lawyers invest in you—they do not receive a dime until your lawsuit reaches a successful resolution!
AFFF Lawsuit claims are being filed against manufacturers of aqueous film-forming foam (AFFF), commonly used in firefighting.
Claims allege that companies such as 3M, DuPont, and Tyco Fire Products failed to adequately warn users about the potential dangers of AFFF exposure — including increased risks of various cancers and diseases.
Depo Provera Lawsuit claims are being filed by individuals who allege they developed meningioma (a type of brain tumor) after receiving Depo-Provera birth control injections.
A 2024 study found that women using Depo-Provera for at least 1 year are five times more likely to develop meningioma brain tumors compared to those not using the drug.
Suboxone Tooth Decay Lawsuit claims are being filed against Indivior, the manufacturer of Suboxone, a medication used to treat opioid addiction.
Claims allege that Indivior failed to adequately warn users about the potential dangers of severe tooth decay and dental injuries associated with Suboxone’s sublingual film version.
Social Media Harm Lawsuits are being filed against social media companies for allegedly causing mental health issues in children and teens.
Claims allege that companies like Meta, Google, ByteDance, and Snap designed addictive platforms that led to anxiety, depression, and other mental health issues without adequately warning users or parents.
Transvaginal Mesh Lawsuits are being filed against manufacturers of transvaginal mesh products used to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
Claims allege that companies like Ethicon, C.R. Bard, and Boston Scientific failed to adequately warn about potential dangers — including erosion, pain, and infection.
Bair Hugger Warming Blanket Lawsuits involve claims against 3M — alleging their surgical warming blankets caused severe infections and complications (particularly in hip and knee replacement surgeries).
Plaintiffs claim 3M failed to warn about potential risks — despite knowing about increased risk of deep joint infections since 2011.
Baby Formula NEC Lawsuit claims are being filed against manufacturers of cow’s milk-based baby formula products.
Claims allege that companies like Abbott Laboratories (Similac) and Mead Johnson & Company (Enfamil) failed to warn about the increased risk of necrotizing enterocolitis (NEC) in premature infants.
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?