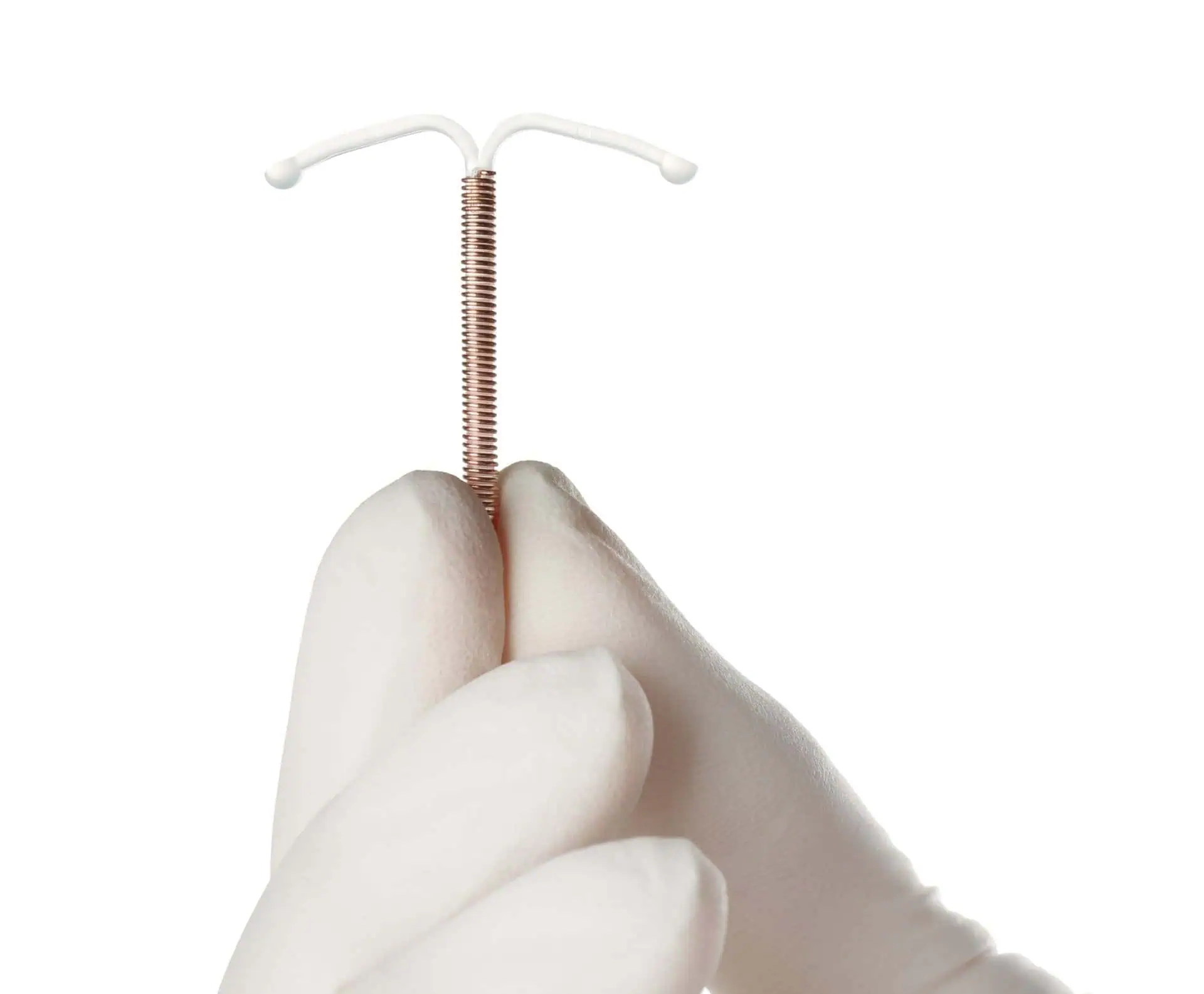
The Paragard IUD is marketed as a simple, safe, hormone free, and effective intrauterine device.
The T-shaped device is made of plastic and then wrapped with copper.
This long-term birth control device is placed in the uterus in minutes during a regular office visit to the doctor and is supposed to last for ten years.
Removal is marketed as a nonsurgical procedure done in just a few minutes during an office visit.
According to the manufacturer, the copper in Paragard interferes with sperm so that it does not reach the egg.
At over 99% effective, Teva claims Paragard prevents pregnancies as well as sterilization.

But, according to a Facebook group with more than 11,000 members, complications due to copper toxicity and breakage of the device happen more frequently than Teva warns women.
According to this Facebook page, the side effects of this medical product are not worth it – these prior users are warning other women, when it comes to Paragard – “Don’t get one”