Mirena Brain Injury Lawsuit
- Last Updated: July 14th, 2025

Attorney Jessica Paluch-Hoerman, founder of TruLaw, has over 28 years of experience as a personal injury and mass tort attorney, and previously worked as an international tax attorney at Deloitte. Jessie collaborates with attorneys nationwide — enabling her to share reliable, up-to-date legal information with our readers.
Legally Reviewed
This article has been written and reviewed for legal accuracy and clarity by the team of writers and legal experts at TruLaw and is as accurate as possible. This content should not be taken as legal advice from an attorney. If you would like to learn more about our owner and experienced injury lawyer, Jessie Paluch, you can do so here.
Fact-Checked
TruLaw does everything possible to make sure the information in this article is up to date and accurate. If you need specific legal advice about your case, contact us by using the chat on the bottom of this page. This article should not be taken as advice from an attorney.
TruLaw is not currently accepting new Mirena cases due to the uncertainty of the litigation.
December 2018 – The majority of the Mirena cases have been filed under an order of consolidation in the Southern District of New York City under Judge Paul A. Engelmayer.
Due to recent rulings by Judge Engelmayer, we are currently unsure if these cases will be able to move forward and are unable to accept new clients at this time.
We will update this page and our clients when we are certain about the future for the Mirena litigation.

In October 2018, Judge Engelmayer ruled to exclude plaintiff’s scientific evidence that Mirena was the cause of injuries claimed by our clients.
In essence, Judge Engelmayer made a ruling showing that he does not believe our experts.
We strongly disagree with this ruling and our Mirena lawyers are working to reverse this decision.
If plaintiffs are unable to reverse this decision, we will need to reconsider a path forward for our current clients.
Studies find a higher than expected number of brain injuries reported to the FDA with Mirena use, prompting hundreds of women to reach out to lawyers to file Mirena brain injury lawsuits.
According to an April 2017 study, Mirena users were over seven times more likely to develop intracranial hypertension or Pseudotumor cerebri (PTC) than other levonorgestrel intrauterine systems.
Table of Contents
What Is The Mirena IUD?
Mirena is a small T-shaped plastic device inserted into the uterus that releases levonorgestrel, an artificial form of the hormone progestin.
Levonorgestrel is a female hormone that can cause changes in the cervix, making it harder for sperm to reach the uterus and harder for a fertilized egg to attach to the uterus.
A levonorgestrel intrauterine system is a plastic device that is placed in the uterus where it slowly releases the hormone to prevent pregnancy for 3 to 5 years.
The Mirena IUD contains 52 mg of levonorgestrel and initially the hormone is releasted at a rate of approximately 20 mcg/day.
This rate then decreases to half that value after 5 years.
According to Mirena’s prescribing information, the device must be removed by the end of the 5th year.
It can be repoaced at the time of the removal with a new device if you want to continue with this contraceptive method.
Mirena FDA Adverse Events
Mirena is a small T-shaped plastic device inserted into the uterus that releases levonorgestrel, an artificial form of the hormone progestin.
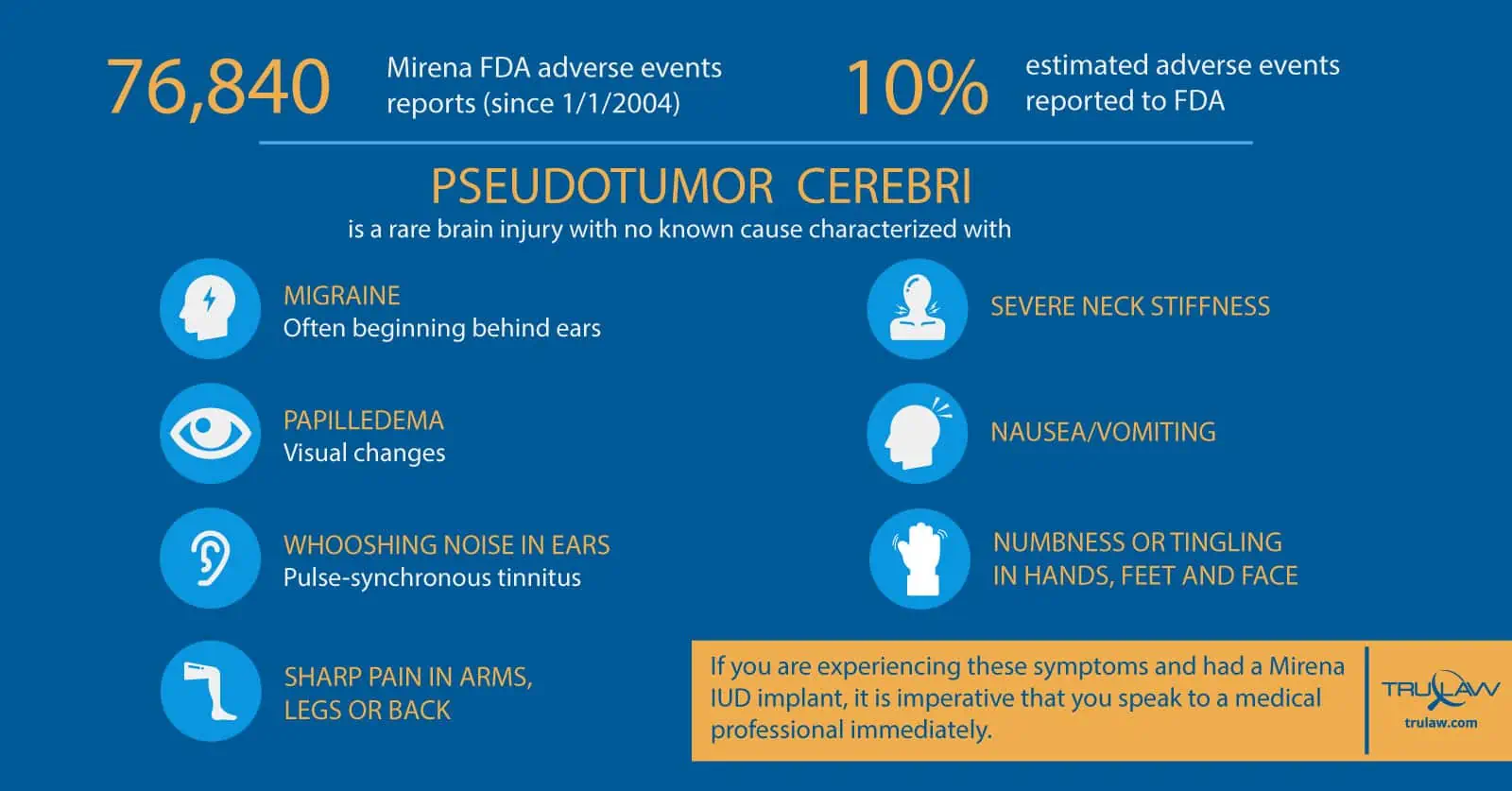
Bayer touts Mirena as “safe and effective” and “well-tolerated by patients” but, in fact, since January 1, 2004 (oldest data available in FDA database), the FDA has received 76,840 adverse event reports related to the Mirena product.
The number of Mirena injuries is likely closer to 700,000 since only about ten percent of all adverse events are actually reported to the FDA.
What Is Idiopathic Intracranial Hypertension Or Pseudotumor Cerebri (PTC)?
Pseudotumor cerebral (PTC) is a syndrome of intracranial hypertension that is idiopathic or from an identified secondary cause.
Idiopathic means a disease that arises spontaneously or with no known cause, as opposed to an identified secondary cause bleeding in the skull or a known event that impacted the brain.
PTC or Idiopathic Intracranial Hypertension (IIH) is generally characterized by headaches and visual loss that may be severe and permanent.
Most Common Symptoms of IIH or Pseudotumor Cerebri
The most common symptoms of IIH or Pseudotumor cerebri are:
- A severe headache beginning behind the ears (a migraine)
- Visual changes (papilledema)
- Whooshing noise in ears (pulse-synchronous tinnitus)
- Vision loss or blindness
- Sharp pain in arms, legs or back
- Severe neck stiffness
- Nausea/vomiting
- Numbness or tingling in hands, feet, and face
- Depression
- Exercise intolerance
- Memory difficulties
IIH affects individuals differently but most women complain of the severe headaches, visual changes, and whooshing noise.
If you are experiencing these symptoms and had a Mirena IUD implant, it is imperative that you speak to a medical professional immediately.
Idiopathic Intracranial Hypertension Diagnosis (Pseudotumor Cerebri Diagnosis)
An Intracranial Hypertension diagnosis (or Pseudotumor cerebri diagnosis) is generally diagnosed as a last resort when doctors can’t find another reason for a series of symptoms in the brain, ears, and eyes.
Some women may ignore the signs for months and consider the symptoms an extreme form of menstrual cycle pain.
But, women of childbearing age with a Mirena IUD, need to be aware of these symptoms and seek a medical professional as soon as possible.
In addition to your medical provider, a diagnosis with IID or pseudotumor cerebri may require two additional medical professionals – a neurologist who will analyze your nervous system and spinal cord and an ophthalmologist to analyze eye problems.
Diagnosis with IIH may require an MRI to review changes in the optic nerve.
While PTC generally occurs in obese women of childbearing age, studies now show that women taking Mirena are over seven times more likely to develop IIH/PTC than other levonorgestrel intrauterine systems such as Skyla, Liletta or Kyleena.
Furthermore, given that levonorgestrel blood levels in women on intrauterine levonorgestrel can fluctuate at times, it is biologically plausible for a woman on Mirena to experience intracranial hypertension after only a few months of use.
Mirena Intracranial Hypertension Lawsuits
Over one hundred lawsuits have been filed against Bayer alleging that the hormonal component in Mirena caused intracranial hypertension.
Since this general causation issue is similar in all cases, on April 6, 2017, the Federal Panel on Multidistrict Litigation granted a petition to “centralize” all of the intracranial hypertension lawsuits into a single federal court.
In re: Mirena IUS Levonorgestrel-Related Products Liability Litigation will be heard in from of the Honorable Paul A. Engelmayer in the Southern District of New York.
This MDL will help facilitate efficient discovery conduct and move the cases forward towards a timelier resolution.
Mirena Settlement
The Mirena Intracranial Hypertension MDL is new, so as of today, none of these cases have proceeded to a jury and there have been no reported settlements of cases, yet.
However, we believe the link between IIH and Mirena is strong and the cases that proceed through the courts will be successful.
It is quite likely that settlement discussions will occur at some point.
Mirena settlements would need to provide adequate compensation for the harm done to women.
These settlements would be confidential and are determined by a number of factors, including the extent of the injury and the strength of the case.

Managing Attorney & Owner
With over 25 years of legal experience, Jessica Paluch-Hoerman is an Illinois lawyer, a CPA, and a mother of three. She spent the first decade of her career working as an international tax attorney at Deloitte.
In 2009, Jessie co-founded her own law firm with her husband – which has scaled to over 30 employees since its conception.
In 2016, Jessie founded TruLaw, which allows her to collaborate with attorneys and legal experts across the United States on a daily basis. This hypervaluable network of experts is what enables her to share the most reliable, accurate, and up-to-date legal information with our readers!
Additional Mirena Brain Injury Lawsuit resources on our website:
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?
At TruLaw, we fiercely combat corporations that endanger individuals’ well-being. If you’ve suffered injuries and believe these well-funded entities should be held accountable, we’re here for you.
With TruLaw, you gain access to successful and seasoned lawyers who maximize your chances of success. Our lawyers invest in you—they do not receive a dime until your lawsuit reaches a successful resolution!
AFFF Lawsuit claims are being filed against manufacturers of aqueous film-forming foam (AFFF), commonly used in firefighting.
Claims allege that companies such as 3M, DuPont, and Tyco Fire Products failed to adequately warn users about the potential dangers of AFFF exposure — including increased risks of various cancers and diseases.
Depo Provera Lawsuit claims are being filed by individuals who allege they developed meningioma (a type of brain tumor) after receiving Depo-Provera birth control injections.
A 2024 study found that women using Depo-Provera for at least 1 year are five times more likely to develop meningioma brain tumors compared to those not using the drug.
Suboxone Tooth Decay Lawsuit claims are being filed against Indivior, the manufacturer of Suboxone, a medication used to treat opioid addiction.
Claims allege that Indivior failed to adequately warn users about the potential dangers of severe tooth decay and dental injuries associated with Suboxone’s sublingual film version.
Social Media Harm Lawsuits are being filed against social media companies for allegedly causing mental health issues in children and teens.
Claims allege that companies like Meta, Google, ByteDance, and Snap designed addictive platforms that led to anxiety, depression, and other mental health issues without adequately warning users or parents.
Transvaginal Mesh Lawsuits are being filed against manufacturers of transvaginal mesh products used to treat pelvic organ prolapse (POP) and stress urinary incontinence (SUI).
Claims allege that companies like Ethicon, C.R. Bard, and Boston Scientific failed to adequately warn about potential dangers — including erosion, pain, and infection.
Bair Hugger Warming Blanket Lawsuits involve claims against 3M — alleging their surgical warming blankets caused severe infections and complications (particularly in hip and knee replacement surgeries).
Plaintiffs claim 3M failed to warn about potential risks — despite knowing about increased risk of deep joint infections since 2011.
Baby Formula NEC Lawsuit claims are being filed against manufacturers of cow’s milk-based baby formula products.
Claims allege that companies like Abbott Laboratories (Similac) and Mead Johnson & Company (Enfamil) failed to warn about the increased risk of necrotizing enterocolitis (NEC) in premature infants.
Here, at TruLaw, we’re committed to helping victims get the justice they deserve.
Alongside our partner law firms, we have successfully collected over $3 Billion in verdicts and settlements on behalf of injured individuals.
Would you like our help?
