Ozempic (semaglutide) represents a glucagon-like peptide-1 (GLP-1) receptor agonist medication that the FDA approved in December 2017 for managing type 2 diabetes.
While initially developed for blood sugar control, this weight management drug and similar medications have gained massive popularity for weight loss purposes, leading to widespread adoption across the United States.

Ozempic (semaglutide) injection 0.5 mg or 1 mg was approved by the FDA in December 2017, and is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
This rapid expansion in use (with nearly 12% of Americans now having tried GLP-1 medications) has brought with it concerning reports of severe side effects that manufacturers allegedly failed to adequately disclose, forming the basis of thousands of lawsuits now consolidated in federal court.
What Is Ozempic and How Does It Work?
Ozempic operates as a prescription medication designed to mimic the naturally occurring GLP-1 hormone that your body produces after food intake.
The FDA approved this once-weekly injection on December 5, 2017, specifically for adults with type 2 diabetes to help regulate blood sugar levels when combined with diet and exercise.
Ozempic is a glucagon-like peptide-1 (GLP-1) analog for the treatment type 2 diabetes.
The medication’s unique molecular structure allows it to remain active in the bloodstream for approximately one week, making it convenient for patients who prefer weekly rather than daily injections.

GLP-1 medications like Ozempic affect multiple body systems through these primary mechanisms:
- Stimulating insulin secretion: When blood glucose levels rise after meals, Ozempic triggers the pancreas to release insulin in a glucose-dependent manner, helping cells absorb sugar from the bloodstream
- Decreasing glucagon release: The medication suppresses the hormone glucagon, which normally signals the liver to release stored glucose, thereby preventing unnecessary increases in blood sugar
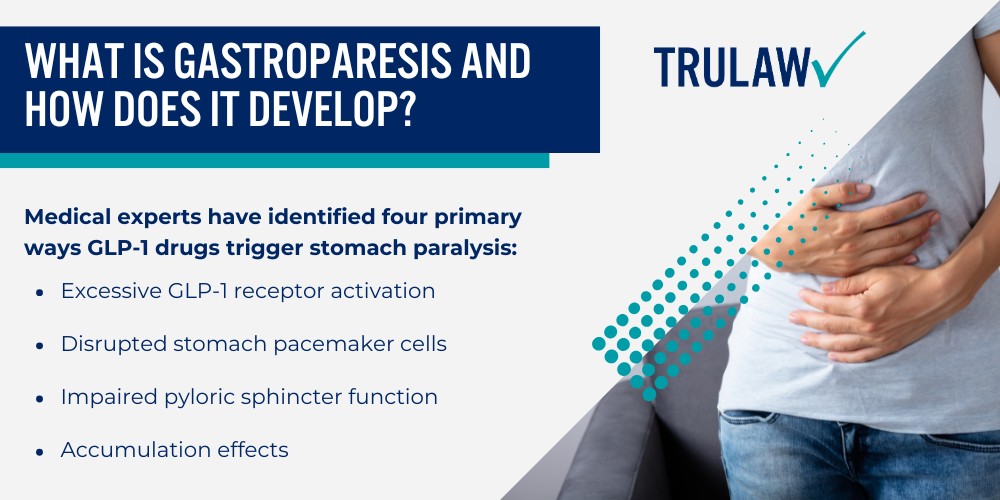
- Slowing gastric emptying: By delaying how quickly food leaves the stomach and enters the small intestine, Ozempic prolongs feelings of fullness and reduces post-meal blood sugar spikes – this same mechanism (which healthcare providers now recognize) has been linked to severe complications in litigation
- Affecting brain receptors: The medication acts on GLP-1 receptors in brain regions that control appetite and satiety, contributing to substantial weight loss effects that led to its widespread off-label use
The FDA approval in December 2017 marked the beginning of what would become one of the fastest-growing pharmaceutical markets in recent history.
Clinical trials demonstrated that Ozempic produced statistically notable reductions in hemoglobin A1c levels compared to placebo and other diabetes medications, with the added benefit of body weight reduction as a secondary outcome.
This weight loss effect, though initially positioned as a side benefit for diabetes patients, quickly became the primary reason many people sought prescriptions.
By August 2025, research from the RAND American Life Panel showed that approximately 11.8% of American adults (nearly 1 in 8 people) had used GLP-1 medications at least once, marking a dramatic increase in adoption.
Novo Nordisk later capitalized on these weight loss effects by releasing Wegovy in June 2021, which contains higher doses of the same semaglutide molecule but carries FDA approval specifically for chronic weight management.
This widespread use of these popular weight loss drugs, both for approved diabetes treatment and off-label weight loss, has now resulted in thousands of patients reporting severe gastrointestinal injuries and vision loss, conditions that many allege were not adequately disclosed on warning labels when they began treatment.
Other GLP-1 Medications Involved in Litigation
The federal multidistrict litigation consolidated as MDL 3094 encompasses multiple GLP-1 receptor agonist medications beyond just Ozempic, reflecting the class-wide nature of the alleged injuries and inadequate warnings.

This class of medications includes:
- Ozempic, Wegovy, and Rybelsus (produced by the Novo Nordisk defendants); and
- Trulicity and Mounjaro (produced by Eli Lilly and Company).
These medications share similar mechanisms of action (all activate GLP-1 receptors to slow gastric emptying and regulate blood sugar) which forms the basis for their consolidation in a single litigation proceeding.
While Ozempic and other GLP-1 medications contain different active molecules and carry distinct brand names, they allegedly cause the same types of severe gastrointestinal complications.
Federal courts have consolidated cases involving these GLP-1 receptor agonist medications:
- Wegovy (semaglutide): Manufactured by Novo Nordisk and approved by the FDA in June 2021 specifically for chronic weight management in adults with obesity or who are overweight with at least one weight-related condition; contains higher doses of semaglutide (up to 2.4 mg weekly) compared to Ozempic’s maximum 2 mg dose
- Rybelsus (oral semaglutide): Also from Novo Nordisk, this represents the first oral GLP-1 medication approved for type 2 diabetes in September 2019; despite being taken as a daily pill rather than injection, it contains the same active ingredient and allegedly carries similar risks
- Mounjaro (tirzepatide): Manufactured by Eli Lilly and approved in May 2022, this medication works as a dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist, offering enhanced blood sugar control and weight loss effects but also allegedly causing severe gastrointestinal injuries similar to semaglutide products
- Trulicity (dulaglutide): Eli Lilly’s once-weekly GLP-1 injection approved in 2014 for type 2 diabetes; while older than Ozempic, it operates through the same gastric-slowing mechanism and faces allegations of inadequate warnings about gastroparesis and related complications
- Saxenda (liraglutide): A Novo Nordisk product approved in 2014 for chronic weight management that requires daily rather than weekly injections; the Judicial Panel on Multidistrict Litigation added Saxenda to MDL 3094 in December 2024, expanding the scope to include this earlier-generation GLP-1 medication
The consolidation of these medications into a single MDL proceedings streamlines the litigation process while allowing each plaintiff to maintain their individual claim for damages.
All five medications share common allegations that their manufacturers knew or should have known about the risks of gastroparesis, intestinal obstruction, and other severe gastrointestinal injuries, yet failed to provide adequate warnings to patients and physicians.
The pharmaceutical companies’ extensive marketing of these drugs (with Novo Nordisk and Eli Lilly commanding market dominance and generating billions in annual revenue) created widespread exposure among both diabetes patients and those seeking weight loss.
The judicial panel concluded that grouping these medications together serves the interests of judicial efficiency because they involve overlapping questions about corporate knowledge, warning label adequacy, and the causal relationship between GLP-1 receptor activation and severe gastric complications.
This multi-defendant, multi-drug approach reflects the systemic nature of the alleged failures to warn, as opposed to isolated problems with a single product.
If you or a loved one developed gastroparesis, intestinal blockages, or other severe gastrointestinal injuries after taking Ozempic, Wegovy, Mounjaro, or similar GLP-1 medications, you may be entitled to compensation.
Contact TruLaw using the chat on this page to receive an instant case evaluation and determine if you qualify to file an Ozempic lawsuit today.