Ozempic has become one of the most prescribed medications in the United States, with millions of patients using it for diabetes management and weight loss purposes.
Recent scientific research has revealed alarming connections between this popular drug and serious vision problems that can lead to permanent blindness.

The emergence of non-arthritic anterior ischemic optic neuropathy (NAION) cases among Ozempic users has prompted widespread concern and legal action against manufacturer Novo Nordisk.
What is Ozempic and How Does It Work?
Ozempic, containing the active ingredient semaglutide, belongs to a class of medications known as glucagon-like peptide-1 (GLP-1) receptor agonists.
The Food and Drug Administration approved Ozempic in December 2017 to treat diabetes, specifically Type 2, marking an advancement in diabetes care.
This injectable medication works by mimicking a natural hormone that regulates blood sugar levels and slows gastric emptying, which helps control appetite and helps patients lose weight.

The GLP-1 receptor agonist works through multiple pathways in the body:
- Stimulating insulin production when blood sugar levels rise
- Reducing glucagon release from the pancreas
- Slowing the movement of food through the digestive system
- Sending signals to the brain that create feelings of fullness
- Regulating appetite centers in the hypothalamus
Related medications containing semaglutide have expanded the drug’s market reach.
Wegovy, approved by the FDA in June 2021 as a weight management drug, provides higher doses specifically for obese and overweight patients.
Rybelsus offers an oral formulation for those who prefer not to use injections, becoming the first oral GLP-1 receptor agonist approved for diabetes treatment.
Market data reveals the extraordinary popularity of these medications, with prescriptions exceeding 3 million annually in 2024 alone.
The pharmaceutical industry reports that approximately 19 million Americans have used GLP-1 medications for diabetes and chronic weight management, generating over $8 billion in annual sales for Ozempic specifically.
This widespread adoption has made semaglutide one of the most financially successful diabetes medications in history.
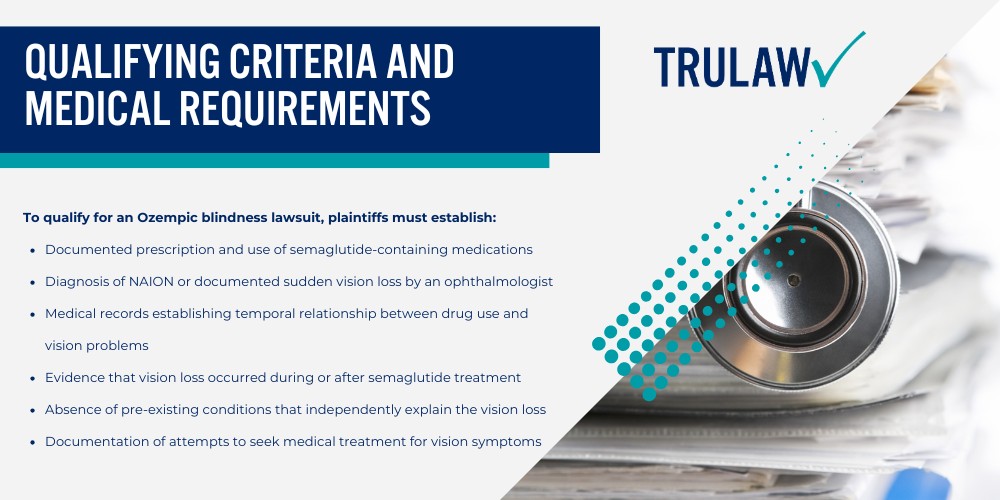
If you or a loved one experienced vision loss or NAION after taking Ozempic, you may be eligible to seek compensation.
Contact TruLaw using the chat on this page to receive an instant case evaluation and determine whether you qualify to join others in filing an Ozempic blindness lawsuit today.
The Emergence of Vision-Related Side Effects
The discovery of serious vision problems associated with Ozempic represents a troubling development that wasn’t apparent during initial clinical trials.
The medical community began noticing unusual patterns of vision loss among their patients using semaglutide medications, particularly cases of NAION that seemed to occur at higher rates than expected in the general population.
These observations raised serious concerns that prompted researchers at Massachusetts Eye and Ear, a Harvard teaching hospital, to conduct a comprehensive investigation.
Researchers documented these milestones in uncovering the vision risks:
- Summer 2023: Three NAION cases presented at Mass Eye and Ear within a short period, all involving semaglutide users
- July 2024: Publication of the landmark Harvard study in JAMA Ophthalmology revealing increased NAION risk
- December 2024: Danish researchers published supporting studies involving over 424,000 patients
- 2025: Multiple international studies confirmed the association between semaglutide and vision problems
The pivotal Harvard study analyzed medical records from 16,827 patients treated between 2017 and 2023, comparing those prescribed semaglutide against patients using other diabetes or weight loss medications.
Researchers discovered that diabetic patients taking semaglutide faced a four-fold increased risk of developing NAION, while those using it for weight management experienced an even more alarming seven-fold increase in risk.
Clinical trials conducted before FDA approval failed to identify these serious vision risks for several reasons.
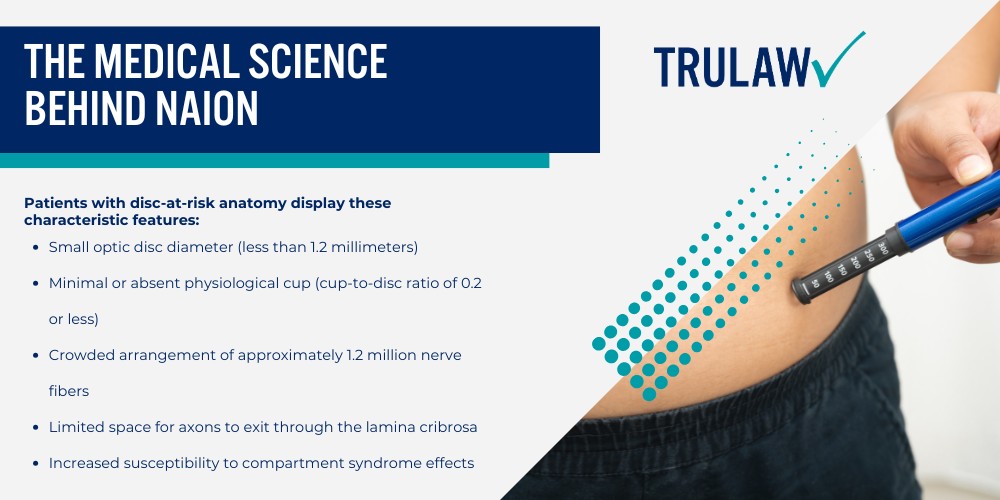
The relatively rare occurrence of NAION in the general population, affecting approximately 10 per 100,000 people over age 50 annually, meant that standard clinical trials weren’t large enough to detect this adverse drug reaction.
Additionally, the condition typically develops months or even years after starting treatment, beyond the timeframe of most pre-approval studies.
The distinction between mild vision changes initially reported and serious NAION cases has become increasingly clear through post-market surveillance.
While some patients experienced temporary blurred vision or minor visual disturbances that resolved without permanent damage, NAION represents a fundamentally different and far more serious condition.
This type of optic nerve damage often results in permanent, irreversible vision loss that impacts patients’ quality of life and ability to work.