Several heater-cooler lawsuits have been filed against the device manufacturer Liva Nova PLC (formerly Sorin Group Deutschland GmbH) on behalf of individuals diagnosed with M. Chimaera after having open-heart surgery.
The lawsuit alleges that the device was designed and manufactured with defects that were recognized as a potential source of infection as early as 2002.
Furthermore, the 3T Stockert heater-cooler system lawsuit alleges that even though Liva Nova PLC knew or should have known of these defects, they failed to warn patients and medical providers of the risk.
In fact, there have been 15 versions of the “instructions for use” (IFU) sent to medical professionals with the Stockert 3T heater-cooler device and none of them have a validated cleaning and disinfection procedure.
Of the 16 heater-cooler lawsuits that have been filed in six different districts, 10 of the cases are being informally coordinated in a South Carolina federal court, and six actions are pending outside of South Carolina.
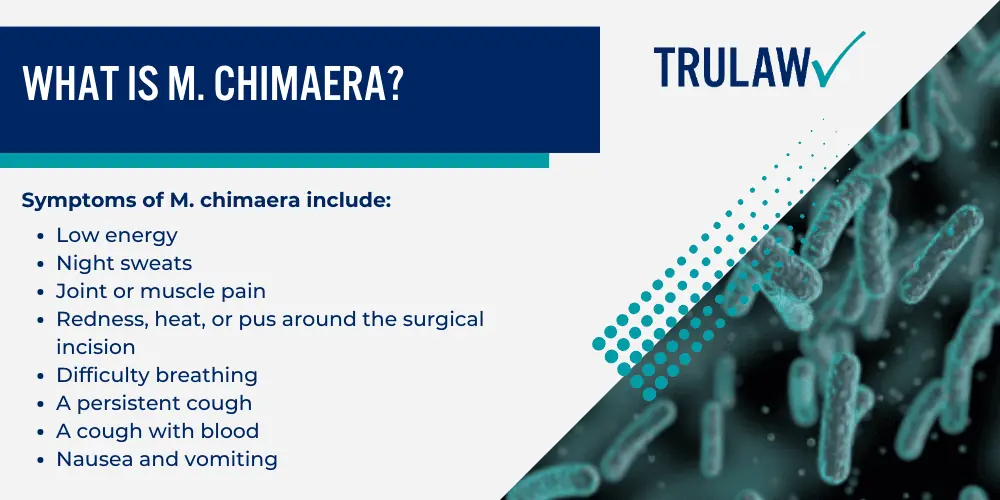
The heater-cooler lawsuits make common allegations that the plaintiffs were exposed to M. chimaera or M. abscessus, types of non-tuberculous mycobacteria (NTM) after undergoing surgery utilizing the 3T Heater-Cooler system.
While some patients do not become ill from NTM exposure, those with a weak immune system may contract a slowly progressive and destructive disease that may become chronic and require ongoing medical treatment.