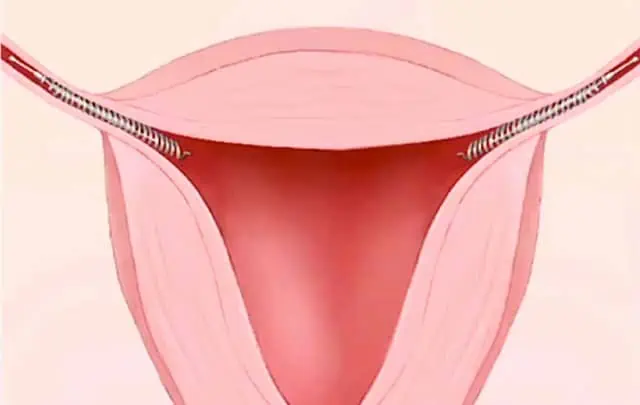
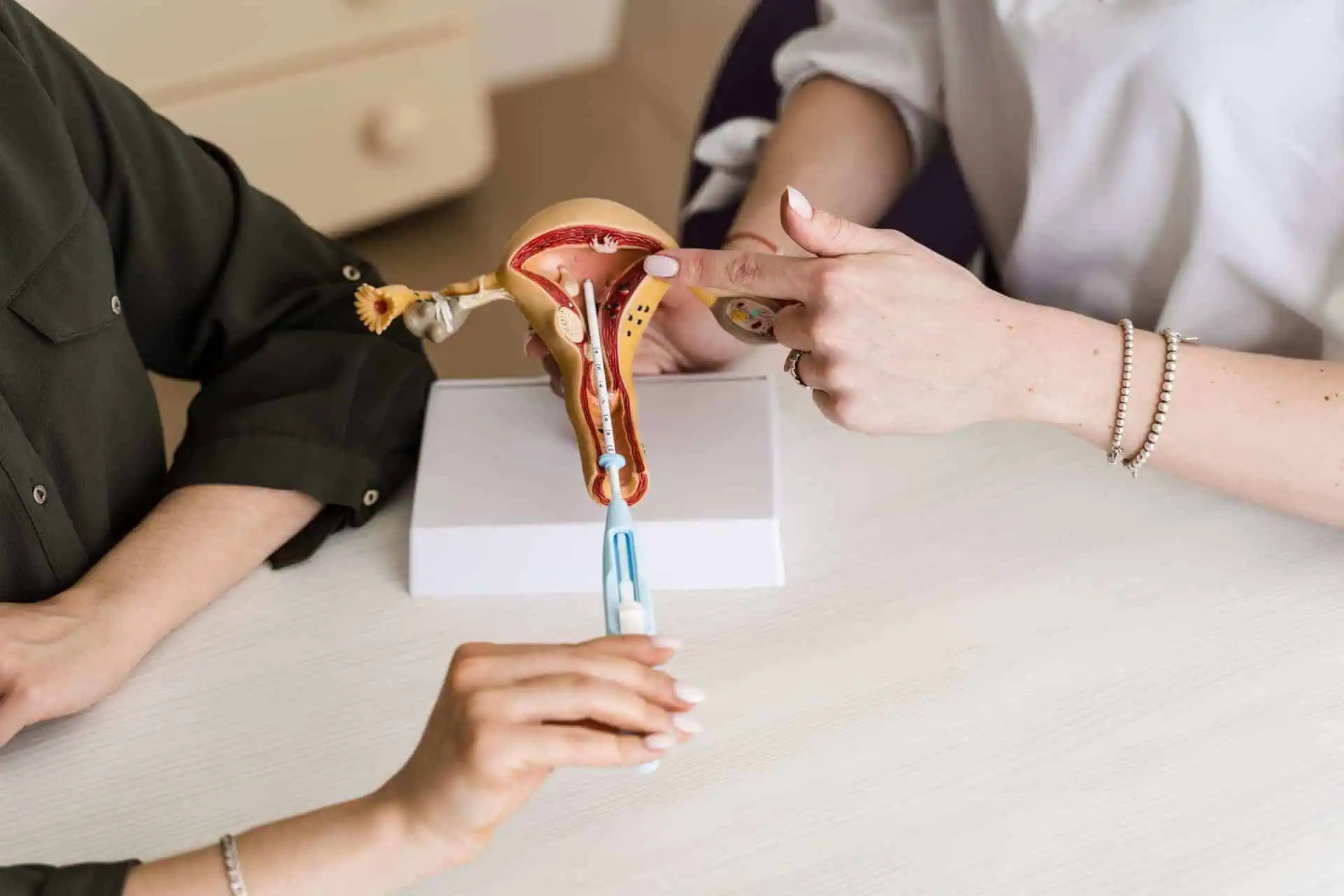
Thousands of Essure lawsuits have been filed against Bayer Inc. (the manufacturer of Essure) by women who have experienced life-changing complications.
Migration/Expulsion of Device
Women report migration of the Essure device outside the uterus and can the implant can get lodged in organs or the abdominal wall.
Essure devices have also been found in the uterus.
Injuries resulting from migration of the Essure device vary – women have found the coils of the device in many different locations in their body, sometimes puncturing organs.
Invasive hysterectomies have been required to remove these devices. Sometimes multiple surgeries are required to remove the devices.
Painful Symptoms / Constellation of Symptoms
Women describe many symptoms that have been explained by their doctors as their bodies learning to accept the coils – some women find the pain does go away but for many women, these symptoms continue and even increase.
Some women describe a constellation of symptoms while their body fights to reject the device in their body.
These symptoms vary tremendously with the women and include bloating/weight gain, pain with sexual intercourse, incontinence, chronic pelvic pain and cramping, heavy periods and more.
Fetal Deaths Under-Reported
Congressman Mike Fitzpatrick (R-PA) recently revealed 303 fetal deaths caused by Essure, as opposed to the 5 deaths reported to the FDA.
On November 4, 2015 he announced the introduction of the E-Free Act — legislation to remove Essure from the market.
According to a private analyst who combed through the FDA’s public database, the FDA may have greatly underestimated the number of fetal deaths among women who became pregnant after using Essure.
The FDA reported a mere five fetal deaths while Madris Tomes, founder, and CEO of Device Events, noted there were 303 reports of fetal deaths tied to Essure as reported in the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database.
Ectopic Pregnancies
Women implanted with Essure have a higher percentage of Ectopic pregnancies.
An ectopic pregnancy cannot support the life of a fetus for very long and risks the health of the mother if immediate care is not sought.
Women with Essure devices have a higher percentage of ectopic pregnancies.
Ectopic pregnancies occur when fertilization of the egg happens outside of the uterus.
Ectopic pregnancies can’t proceed normally.
Nickel Injuries/Allergies
Originally, Conceptus warned women to test for nickel allergies prior to Essure implant.
But, proudly had this warning and the required skin test removed from the label in 2011.
Many women describe allergy-like reactions to Nickel that include hair loss, dental issues, weight gain, fatigue and more.
Essure is 55% nickel – Many women report side effects that may actually be reactions to nickel in their body.
Reported side effects that may be an allergic reaction to nickel include hair loss, dental issues, weight gain, chronic fatigue, and more.
The original Essure label required a skin test and warned of nickel allergies, this was removed in 2011.