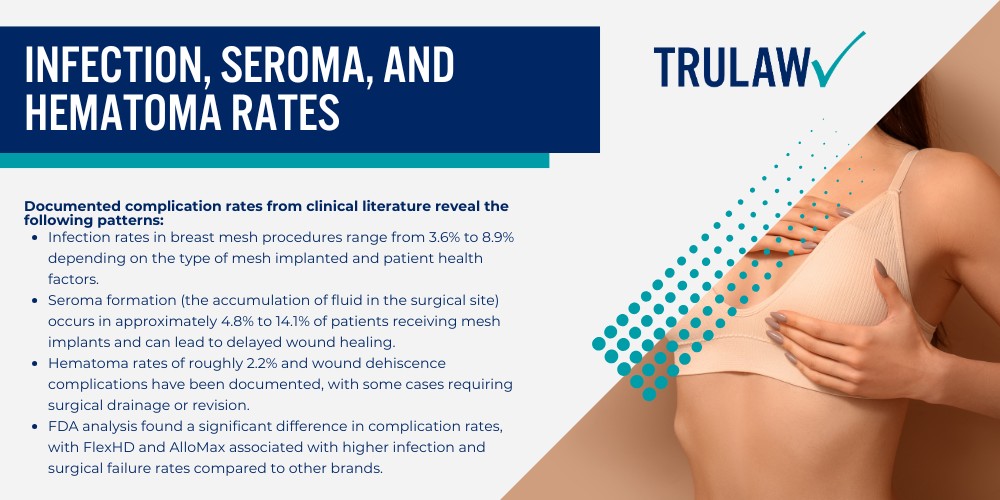
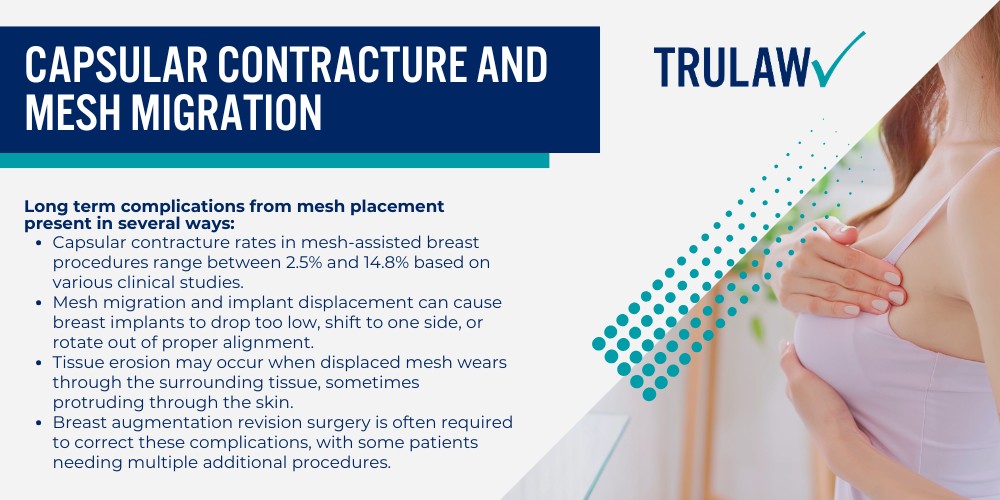
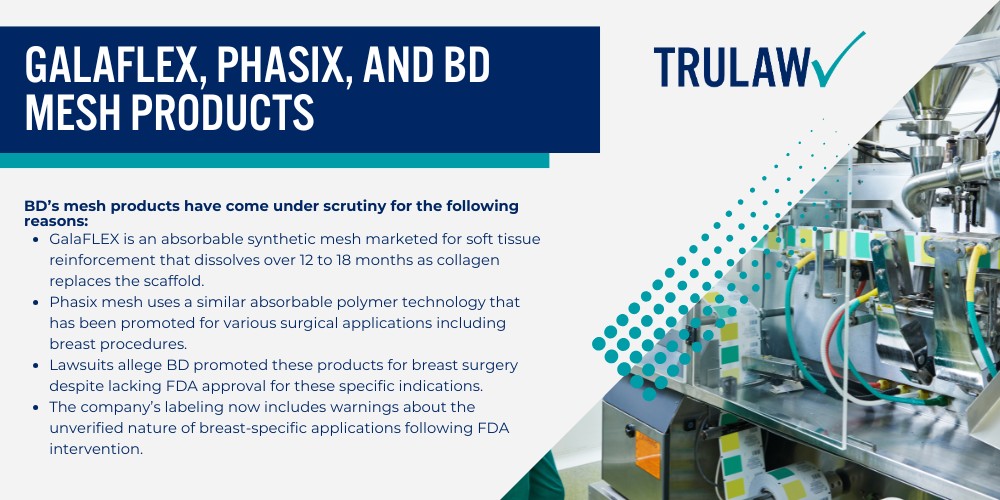
The internal bra breast lift technique involves placing a supportive mesh material beneath the breast tissue to create a permanent internal support structure that mimics the function of a push up bra.
A board certified plastic surgeon developed this approach to address sagging breasts and maintain the position of implants or natural tissue in cases where a regular breast lift would not provide lasting results.

During the procedure, the surgeon attaches the mesh scaffold to the chest wall along the inframammary fold (the crease where the breast meets the torso) to provide extra support.
The internal bra offers soft tissue support and structural reinforcement to the lower pole and upper pole of the breast pocket while the body’s natural collagen formation eventually integrates with the material over a period of 12 to 18 months.
Types of Biologic and Synthetic Meshes Used in Internal Bra Procedures
Two primary categories of internal mesh materials have been used in internal bra procedures: biologic matrices derived from human or animal tissue and synthetic meshes made from absorbable or non-absorbable polymers.
The choice of mesh materials often depends on the specific surgical application, patient characteristics, and surgeon preference.

Four primary mesh materials have been deployed in internal bra procedures:
- Human-derived acellular dermal matrix (ADMs) include products such as AlloDerm, AlloMax, FlexHD, and DermaMatrix, which are processed from donated human tissue with cells removed while leaving the collagen scaffold intact.
- Animal-derived ADMs derive from Strattice (porcine), SurgiMend (bovine), and Permacol, which undergo processing to remove cellular components and fatty tissue while preserving the structural framework.
- Absorbable mesh products like GalaFLEX, Phasix, TIGR Matrix, and Vicryl are designed to dissolve over time as the body replaces them with natural scar tissue.
- Non-absorbable synthetic meshes including TiLOOP Bra and SERAGYN BR remain permanently in the body after implantation.
These materials vary in their integration with surrounding breast tissue, their potential for complications over time, and their impact on aesthetic outcomes.
Identifying which specific mesh product was used during surgery is often a key factor in determining eligibility for legal action.
Why Breast Mesh Implants Are Used in Breast Augmentation and Reconstruction
Surgeons began incorporating mesh into breast procedures to provide additional tissue reinforcement for weak breast tissue and implant support that a traditional breast lift alone could not achieve.
The mesh allows plastic surgeons to accomplish results that would otherwise require mobilizing substantial amounts of the patient’s own tissue.
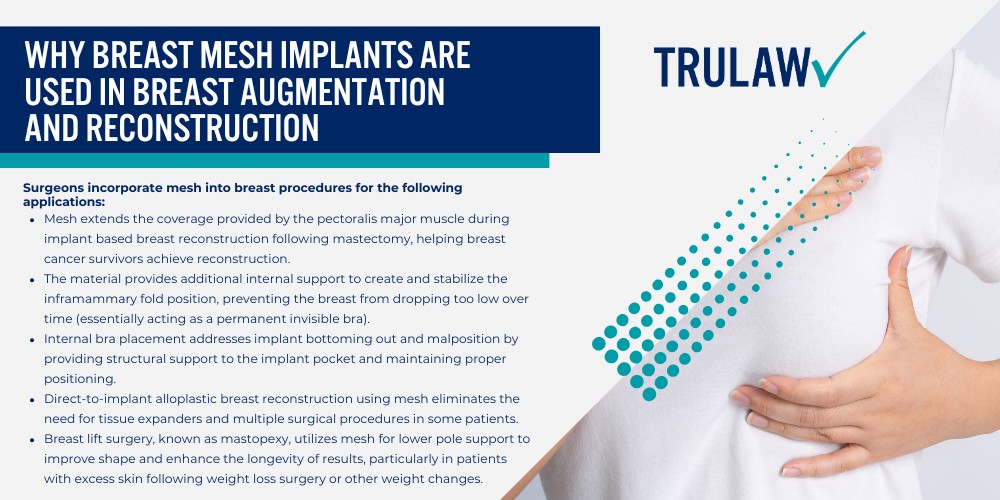
Surgeons incorporate mesh into breast procedures for the following applications:
- Mesh extends the coverage provided by the pectoralis major muscle during implant based breast reconstruction following mastectomy, helping breast cancer survivors achieve reconstruction.
- The material provides additional internal support to create and stabilize the inframammary fold position, preventing the breast from dropping too low over time (essentially acting as a permanent invisible bra).
- Internal bra placement addresses implant bottoming out and malposition by providing structural support to the implant pocket and maintaining proper positioning.
- Direct-to-implant alloplastic breast reconstruction using mesh eliminates the need for tissue expanders and multiple surgical procedures in some patients.
- Breast lift surgery, known as mastopexy, utilizes mesh for lower pole support to improve shape and enhance the longevity of results, particularly in patients with excess skin following weight loss surgery or other weight changes.
Despite these intended benefits, the FDA has not approved any mesh products specifically for cosmetic breast surgery or reconstructive surgery applications, meaning these uses remain off-label with unverified safety profiles.
If you or a loved one received a breast mesh implant and experienced complications, you may have legal options to pursue compensation for your injuries.
Contact TruLaw using the chat on this page to receive an instant case evaluation and learn whether you qualify to file a Breast Mesh lawsuit today.