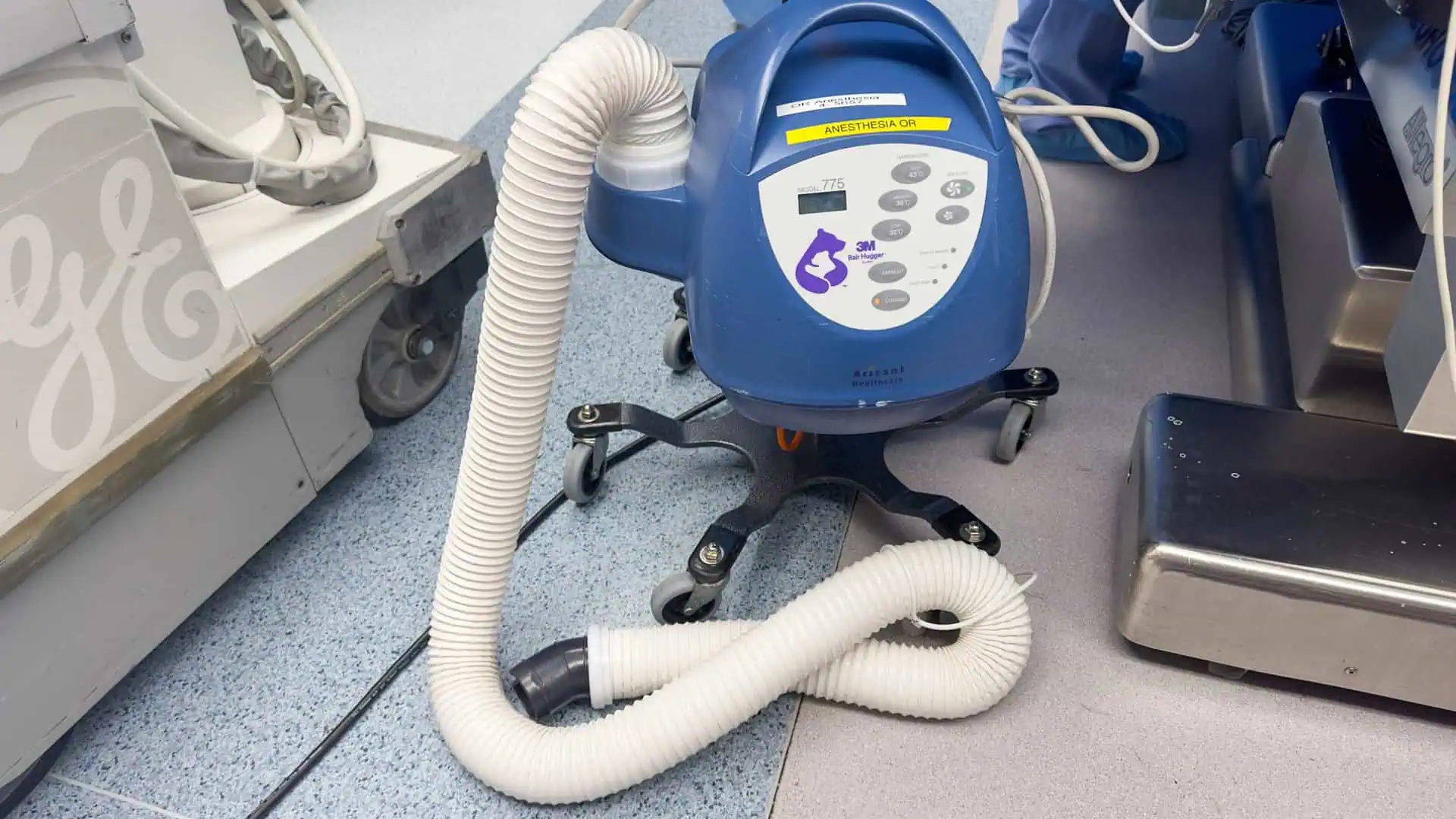
The 3M Bair Hugger Warming System is a forced-air patient warming device designed to maintain normothermia (normal body temperature) in patients before, during, and after surgery.
Developed by 3M Company and its subsidiary Arizant Healthcare, these Bair Hugger devices have become widely used tools in operating rooms across the United States.
The Bair Hugger system consists of a warming unit that generates warm air that’s distributed through a disposable blanket placed over the patient.
This method of warming is intended to help prevent perioperative hypothermia, a condition where a patient’s body temperature drops below normal during surgery.
Nationwide Use of Bair Hugger Blankets
The Bair Hugger Warming System has achieved significant market penetration in the United States healthcare system:
- It is reportedly used by more than 80% of U.S. hospitals.
- The system has become the number one choice for patient warming in the United States.
- Its widespread adoption is due in part to its ease of use and perceived effectiveness in maintaining patient body temperature during surgical procedures.
However, the Bair Hugger blanket has also been controversial due to its association with increased infection risks during surgeries.
This extensive use across the country underscores the system’s importance in modern surgical practices and highlights the potential impact of the ongoing litigation.
Factors Contributing to Bair Hugger Infection Risk
Despite its widespread use, the Bair Hugger forced-air system has come under scrutiny due to concerns about potential infection risks.
Several factors have been identified as potentially contributing to these risks:
- Airflow Disruption: Some studies suggest that the forced-air warming system may disrupt the clean airflow in operating rooms, potentially introducing contaminants to the surgical site.
- Bacteria Spread: Critics argue that the system might spread bacteria from the floor of the operating room to the surgical site, increasing the risk of infection.
- Device Design: The design of the warming unit and the disposable blankets has been questioned, with some experts suggesting that it may not adequately prevent the spread of airborne contaminants.
- Surgical Site Vulnerability: The risk is thought to be particularly high in procedures involving implants, such as hip and knee replacements, where even a small number of bacteria can lead to severe infections.
- Duration of Use: Some research indicates that longer surgeries using the Bair Hugger system may increase the risk of infection, although this finding is not universally accepted.
It’s important to note that the scientific community is divided on these issues.
While some studies have suggested an increased risk of infection associated with the use of forced-air warming devices like the Bair Hugger system, other research, including studies cited by 3M, indicates that the system may help reduce the risk of surgical site infections by maintaining normothermia.
The conflicting nature of these findings is at the heart of the ongoing litigation and continues to be a subject of debate among medical professionals and researchers.